This blog is your ultimate guide to Chapter 8—packed with model test questions, solved examples, and step‑by‑step explanations. Whether you’re preparing for Karachi Board exams, practice tests, or final assessments, you’ll master reactions, mechanisms, and problem‑solving techniques to boost your grades. 🚀
- Intro: Alkyl Halides and Amines—structure, reactivity, and synthesis. 🔬
- Importance: Core building blocks in organic synthesis & pharmaceuticals. 💊
- Applications: Substitution, elimination, and functional group transformations. ⚙️
- Nomenclature: IUPAC names for aldehydes & ketones. 🏷️
- Types: Distinguish aldehydes vs ketones by carbonyl position. 🧩
- Properties: Physical (bp, solubility) & chemical (reactivity trends). 📈
- Reactions: Nucleophilic addition, oxidation, reduction, condensation. 🔄
- MCQs: With answers & quick explanations. ✅
- Short answers: Step by step solutions for exam precision. ✍️
- Numericals: Included where applicable for practice. 🔢
- Nucleophilic addition: Mechanism, stereochemistry, rate factors. 🧭
- Aldol condensation: Formation of β‑hydroxy carbonyls. 🧪
- Oxidation & reduction: Key transformations of carbonyl group. ⚗️
- Decision trees: Identify aldehyde vs ketone reactivity. 🌳
- Reaction maps: From carbonyls to alcohols, acids, derivatives. 🗺️
- Error traps: Common pitfalls & exceptions. ⚠️
- Mechanisms first: Understand nucleophilic addition & condensation steps. 🧠
- Exceptions: Note rearrangements, steric effects, and oxidizing agents. 🧩
- Past papers: Practice Karachi Board patterns. 📚
- Nomenclature: Revise aldehyde vs ketone naming rules. 🏷️
- PDF: Chapter 8 model test questions—free download. ⬇️
- Revision sheet: Aldehydes & Ketones—at‑a‑glance formulas & flows. ⚡
“Struggling with Aldehydes and Ketones? Let’s simplify it with solved model test questions!”
✏️ Model Test Questions XII Chemistry Chapter # 8………… Aldehydes and Ketones ✏️
✏️ Short Questions of Aldehydes and Ketones ✏️
(i) The boiling point of aldehydes and ketones is lower than alcohol and carboxylic acids.
(ii) Formaldehyde is highly soluble in water as compared to other aldehydes.
(iii) Oxidation of aldehydes is faster than ketones.
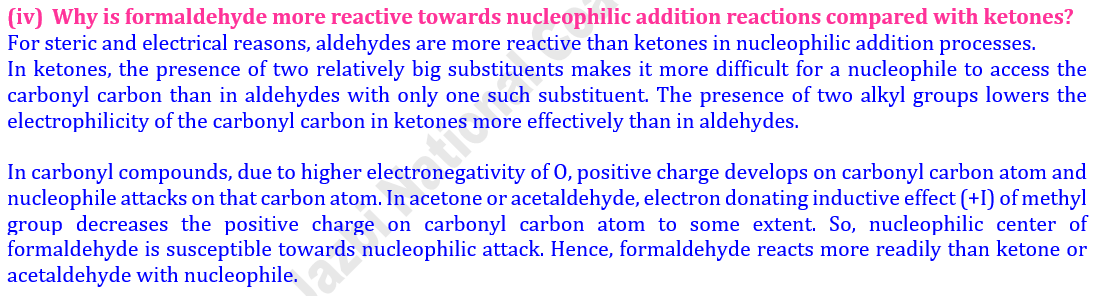
(iv) Why is formaldehyde more reactive towards nucleophilic addition reactions compared with ketones?
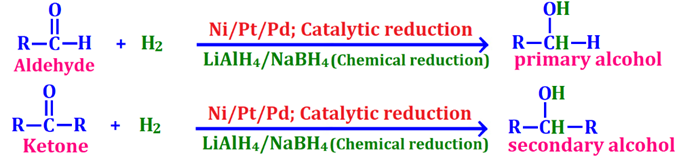
(ii) Lithium aluminium hydride (LiAlH₄)
(iii) Zinc mercury amalgam
(a) Hydrazine
(b) Hydroxylamine
(iii) Hydrogen cyanide
1. H₃C–CHO + HCN → H₃C–HC(CN)OH
2. H–CHO + [O] → HCOOH
3. H₃C–CO–CH₃ + NH₂NHC₆H₅ → (H₃C)₂–C=N NHC₆H₅ + H₂O
4. H–CHO + NH₂OH → H–CH=NOH + H₂O
5. C₆H₆ + CH₃COCl₂ → C₆H₅OCCH₃ + HCl
✏️ Descriptive Questions ✏️
(i) Tollen’s reagent
(ii) Fehling solution
i. Oxidation of acetone with acidified K₂Cr₂O₇
ii. Reduction of acetaldehydes with NaBH₄
iii. Hydration of ethyne
iv. Acylation of benzene in the presence of AlCl₃
i. Hydrogen cyanide
ii. Primary alcohol
iii. Methyl magnesium bromide
iv. Ammonia
✏️ Text Book and Past Papers MCQs on Aldehyde and Ketone ✏️
Q1. Ketones when treated with LiAlH₄, they reduce to
✔ Correct: (b) Secondary alcohol → LiAlH₄ reduces ketones to secondary alcohols.
Q2. The reagent used to oxidize ketones into carboxylic acid is
✔ Correct: (b) Potassium dichromate → strong oxidizing agent converts ketones to acids.
Q3. The carbonyl carbon of aldehydes and ketones is
✔ Correct: (b) sp²-hybridized → carbonyl carbon has trigonal planar geometry.
Q4. Acetophenone is the member of ketone family, it contains:
✔ Correct: (c) One alkyl & one aryl group → acetophenone has C₆H₅–CO–CH₃ structure.
Q5. The most reactive molecules towards nucleophilic addition in the following is
✔ Correct: (a) Formaldehyde → least steric hindrance and most electrophilic carbonyl.
Q6. Clemmensen reduction is the conversion of aldehydes and ketones into
✔ Correct: (a) Alkanes → Clemmensen reduction reduces carbonyl compounds to alkanes using Zn-Hg/HCl.
Q7. Hydration of propyne in the presence of H₂SO₄ and HgSO₄ gives
✔ Correct: (c) Acetone → hydration of propyne follows Markovnikov’s rule, yielding acetone.
Q8. Which of the following carbonyl compounds is most soluble in water?
✔ Correct: (a) Formaldehyde → smallest molecule, strong hydrogen bonding, highest solubility in water.
Q9. Which of the following gives silver mirror test with Tollen’s reagent?
✔ Correct: (a) HCHO → formaldehyde is an aldehyde, gives positive Tollen’s silver mirror test.
Q10. On reduction of carbonyl compound by Zn-Hg and conc. HCl, it is converted to an alkane. This reaction is known as
✔ Correct: (c) Clemmensen reduction → Zn-Hg/HCl reduces carbonyls to alkanes.
Q11. Which one of the following is used in the preparation of antipolio vaccine?
✔ Correct: (d) Formaldehyde → used to inactivate viruses in vaccine preparation.
Q12. Which of the following reagents will react with both aldehydes and ketones?
✔ Correct: (a) 2,4-dinitrophenylhydrazine → forms hydrazones with both aldehydes and ketones.
Q13. The class of organic compounds that are reduced to primary alcohols and respond to Fehling’s solution are known as:
✔ Correct: (c) Aliphatic aldehydes → reduce to primary alcohols and give positive Fehling’s test.
Q14. Ketones are prepared by the oxidation of:
✔ Correct: (b) Secondary alcohols → oxidation yields ketones.
Q15. Aldehydes are prepared by the oxidation of:
✔ Correct: (b) Primary alcohols → oxidation yields aldehydes.
Q16. Which of the following reagents will react with both aldehydes and ketones?
✔ Correct: (d) Phenylhydrazine → reacts with both aldehydes and ketones forming hydrazones.
Q17. The class of organic compounds that are reduced to primary alcohols and respond to Fehling’s solution are known as:
✔ Correct: (d) Aliphatic aldehydes → reduce to primary alcohols and give positive Fehling’s test.
Q18. Which one of the following reagents used to distinguish acetophenone and benzophenone?
✔ Correct: (d) Alkaline iodine solution → acetophenone gives iodoform test, benzophenone does not.
Q19. Which one of the following reagents will distinguish between benzaldehyde and acetophenone?
✔ Correct: (b) Aqueous diamminesilver(I) ions → benzaldehyde gives silver mirror test, acetophenone does not.
Q20. Formalin is:
✔ Correct: (d) 40% solution of formaldehyde in water → standard composition of formalin.
Q21. Aldehydes and ketones are prepared by the controlled oxidation of primary and secondary alcohols respectively by ……….
✔ Correct: (a) Pyridinium chlorochromate → PCC selectively oxidizes primary alcohols to aldehydes and secondary alcohols to ketones.
Q22. Which type of alcohols cannot undergo oxidation?
✔ Correct: (c) Tertiary alcohols → lack hydrogen on the carbon bearing –OH, so they resist oxidation.
Q23. Pyridinium chlorochromate (PCC) is a yellow-orange salt with the formula.
✔ Correct: (a) [C₅H₅NH]⁺[CrO₃Cl]⁻ → actual formula of PCC.
Q24. PCC is a milder version of
✔ Correct: (a) Chromic acid → PCC is a milder oxidant compared to chromic acid.
Q25. Which of the following are more prone to oxidation?
✔ Correct: (a) Aldehydes → easily oxidized to carboxylic acids, unlike ketones.
Q26. The unstable intermediate formed during acid-catalyzed hydration of alkyne is
✔ Correct: (a) Enol → unstable intermediate formed during hydration of alkynes.
Q27. The unstable intermediate enol (alkenol) formed during acid-catalyzed hydration of alkyne undergoes molecular rearrangement giving an aldehyde or ketone. This molecular rearrangement is known as
✔ Correct: (c) Tautomeric change or Tautomerization → enol rearranges to aldehyde or ketone.
Q28. Which of the following alkynes give ketone upon acid-catalyzed hydration?
✔ Correct: (d) All of them → hydration of internal and terminal alkynes yields ketones.
Q29. Acetone is obtained by the acid-catalyzed hydration of
✔ Correct: (a) Propyne → hydration yields acetone.
Q30. The ozonolysis of an alkene initially gives an unstable intermediate called
✔ Correct: (a) Ozonide → unstable intermediate formed in ozonolysis.
Q31. Ozonolysis of ethene gives
✔ Correct: (a) Formaldehyde → ozonolysis of ethene produces two molecules of formaldehyde.
Q32. The carbonyl carbon of carbonyl groups in aldehydes and ketones act as a/an
✔ Correct: (a) Electrophiles → carbonyl carbon is electron-deficient and attracts nucleophiles.
Q33. Aldehydes are more reactive than ketones due to the
✔ Correct: (c) Both of them → aldehydes have less steric hindrance and stronger +I effect making them more reactive.
Q34. During nucleophilic addition reactions, nucleophiles attack the carbonyl carbon of aldehydes and ketones, leading to a change in the hybridization of the carbon atom from
✔ Correct: (a) sp² to sp³ → nucleophilic attack converts trigonal planar carbonyl carbon to tetrahedral.
Q35. During nucleophilic addition reactions, nucleophiles attack the carbonyl carbon of aldehydes and ketones forming a tetrahedral intermediate called
✔ Correct: (a) Alkoxide → nucleophilic addition forms an alkoxide intermediate.
Q36. An acid catalyst is used in a case where a carbonyl compound reacts with
✔ Correct: (a) Weak nucleophile → acid protonates carbonyl to enhance electrophilicity for weak nucleophiles.
Q37. Acid-catalyzed Nucleophilic Addition Reactions of carbonyl compounds is initiated by the attack of
✔ Correct: (c) Acidic proton → protonates carbonyl oxygen, activating carbonyl carbon.
Q38. The intermediate formed during Acid-catalyzed Nucleophilic Addition Reactions of carbonyl compounds is
✔ Correct: (a) Protonated carbonyl group → formed as intermediate in acid-catalyzed addition.
Q39. The protonation of carbonyl group during Acid-catalyzed Nucleophilic Addition Reactions of carbonyl compounds enhances ………… carbonyl carbon
✔ Correct: (a) Electrophilicity → protonation makes carbonyl carbon more susceptible to nucleophilic attack.
Q40. Which of one of the following addition of reagents in carbonyl compounds are acid-catalyzed
✔ Correct: (d) All of them → acid catalysis helps nucleophilic addition of these reagents to carbonyls.
Q41. Which of one of the following addition of reagents in carbonyl compounds are acid-catalyzed
✔ Correct: (d) All of them → acid catalysis helps nucleophilic addition of hydroxylamine, alcohols, and amines to carbonyls.
Q42. The Acid-catalyzed Nucleophilic Addition Reactions of carbonyl compounds are the examples of ……… reaction
✔ Correct: (c) Addition-Elimination → acid-catalyzed nucleophilic addition is a classic addition-elimination mechanism also called Condensation-Elimination.
Q43. The Acid-catalyzed Nucleophilic Addition Reactions of carbonyl compounds converts C=O into
✔ Correct: (a) C=N → imine formation occurs when nucleophiles like amines add to carbonyls.
Q44. The addition of hydrazine to carbonyl compounds gives
✔ Correct: (a) Hydrazone → hydrazine reacts with carbonyls to form hydrazones.
Q45. Addition of hydrazine to carbonyl compounds is an ……….. reaction.
✔ Correct: (a) Acid-catalyzed → hydrazine addition to carbonyls is facilitated by acid catalysis.
Q46. The systematic IUPAC name of hydrazine is
✔ Correct: (c) Diazan/Diamine → official IUPAC name of hydrazine.
Q47. Hydrazine is an inorganic compound and it is a simple pnictogen
✔ Correct: (a) Hydride → hydrazine is a pnictogen hydride.
Q48. The chemical formula of hydrazine is
✔ Correct: (c) Both of them → N₂H₄ and NH₂NH₂ represent hydrazine.
Q49. Hydrazone on reacting with KOH at 200°C gives
✔ Correct: (a) Alkane → Wolff–Kishner reduction converts carbonyls via hydrazones to alkanes.
Q50. The reduction of aldehyde and ketone into alkane by using hydrazine (NH₂–NH₂) and potassium hydroxide is known as
✔ Correct: (a) Wolff–Kishner reaction → uses hydrazine and base to reduce carbonyls to alkanes.
Q51. Addition of hydroxylamine to carbonyl compounds is an ……….. reaction.
✔ Correct: (a) Acid-catalyzed → hydroxylamine addition to carbonyls is facilitated by acid catalysis.
Q52. The addition of hydroxylamine to carbonyl compounds gives
✔ Correct: (b) Oxime → hydroxylamine reacts with carbonyls to form oximes.
Q53. The chemical formula of hydroxylamine is
✔ Correct: (a) NH₂OH → hydroxylamine formula.
Q54. A base catalyst is used in a case where a carbonyl compound reacts with
✔ Correct: (a) Strong nucleophile → base catalysis helps strong nucleophiles attack carbonyl carbon.
Q55. Base-catalyzed Nucleophilic Addition Reactions of carbonyl compounds is initiated by the attack of
✔ Correct: (c) Base → initiates nucleophilic addition by generating nucleophilic species.
Q56. The intermediate formed during base-catalyzed nucleophilic addition reactions of carbonyl compounds is
✔ Correct: (b) Alkoxide ion → formed as intermediate in base-catalyzed addition.
Q57. Base-catalyzed Nucleophilic Addition Reactions of carbonyl compounds gives addition product which is obtained in the form of
✔ Correct: (a) Racemic mixture → nucleophilic addition to planar carbonyl carbon yields racemic products.
Q58. Which one the following is/are base-catalyzed Nucleophilic addition Reactions?
✔ Correct: (c) Both of them → HCN and Grignard’s reagent additions are base-catalyzed nucleophilic additions.
Q59. Addition of hydrogen cyanide to carbonyl compounds gives
✔ Correct: (c) Both of them → HCN addition yields cyanohydrins, also called α-hydroxyalkanenitriles.
Q60. Tollen’s reagent is:
✔ Correct: (c) Ammonical silver nitrate → Tollen’s reagent used for silver mirror test.
✏️ Smart Answers of Short Questions of Aldehydes and ketones✏️
Q1. Give reasons for the following:
(i) The boiling point of aldehydes and ketones is lower than alcohol and carboxylic acids.
👉 Aldehydes and ketones only have dipole–dipole forces of moderate strength. They do not form intermolecular hydrogen bonds among themselves, while alcohols and carboxylic acids do. Weaker intermolecular forces = lower boiling point 🔥⬇️
(ii) Formaldehyde is highly soluble in water compared to other aldehydes.
👉 Formaldehyde is very small in size and easily forms strong hydrogen bonds with water, so it dissolves readily 💧🤝
(iii) Oxidation of aldehydes is faster than ketones.
👉 Aldehydes have a hydrogen atom attached to the carbonyl carbon, which is easily oxidized, whereas ketones lack this hydrogen and resist oxidation. The oxidation of ketone to acid involves cleavage of stronger C–C bond which is difficult and requires strong drastic conditions ⚡🧪
(iv) Formaldehyde is more reactive towards nucleophilic addition than ketones.
👉 Formaldehyde has no alkyl groups, so there is less steric hindrance and no +I (electron donating inductive effect), making the carbonyl carbon more electrophilic and easily attacked by nucleophiles 🎯✨
Q2. Write the equation for the reaction of acetaldehyde with the following:
(i) Reaction with Chromic acid (H₂CrO₄)
👉 Acetaldehyde gets oxidized to acetic acid
⚗️ Equation: CH₃CHO + [O] —H₂CrO₄→ CH₃COOH ✨ Oxidation reaction 🔥
(ii) Reaction with Lithium aluminium hydride (LiAlH₄)
👉 Acetaldehyde is reduced to ethanol
⚗️ Equation: CH₃CHO + H₂ —LiAlH₄/ether→ CH₃CH₂OH ⬇️ Reduction reaction
(iii) Reaction with Zinc–mercury amalgam (Zn–Hg / HCl)
👉 Clemmensen reduction: carbonyl group → methylene group
⚗️ Equation: CH₃CHO + 4[H] —Zn-Hg/HCl→ CH₃CH₃ + H₂O 🔩 Strong reduction → alkane
Q3. What is ozonolysis? How would you obtain formaldehyde and acetone from ozonolysis of alkenes?
Definition:
Ozonolysis is a chemical reaction in which an alkene reacts with ozone (O₃), leading to the cleavage of the C=C double bond. The reaction proceeds via formation of an unstable ozonide, which on decomposition in the presence of a reducing agent (Zn/H₂O) or oxidizing agent (H₂O₂) gives aldehydes and ketones ⚡🧪
👉 Formaldehyde is obtained by ozonolysis of ethene
👉 Acetone is obtained by ozonolysis of 2,3-dimethylbut-2-ene
⚗️ General Reaction: Alkene + O₃ —Ether→ Ozonide —Zn/H₂O (100°C)→ Aldehydes / Ketones
📌 Thus, ozonolysis is used to identify the position of double bonds and to prepare carbonyl compounds.
Q4. How does the oxidation of ketones differ from the oxidation of aldehydes?
Aldehydes:
Aldehydes are easily oxidized because they contain an active hydrogen atom attached to the carbonyl carbon. This hydrogen can be readily removed, allowing aldehydes to be oxidized by mild oxidizing agents such as Tollens’, Fehling’s, Benedict’s reagents, and chromic acid (H₂CrO₄) ⚡🧪
👉 No breaking of the C–C bond is required, so oxidation is fast and easy.
Ketones:
Ketones, on the other hand, are resistant to oxidation because they lack an active (acidic) hydrogen on the carbonyl carbon. Oxidation of ketones requires strong oxidizing agents like acidified potassium dichromate (K₂Cr₂O₇) 🔥
👉 This process involves breaking a C–C sigma bond, making oxidation slow and difficult. Ketones are oxidized to lower carboxylic acids with fewer carbon atoms, often with the loss of one carbon atom as CO₂ 💨
📌 In summary:
✨ Aldehydes → No breaking of the C–C bond → easily oxidized → mild oxidizing agents → carboxylic acids
✨ Ketones → breaking of the C–C bond → difficult to oxidize → require strong oxidants → lower carboxylic acids
Q5. What reagent can be used to convert an alcohol to aldehydes and ketones without the formation of carboxylic acid? OR How do you prepare carbonyl compounds by controlled Oxidation of primary alcohols and secondary alcohols
Controlled Oxidation:
Aldehydes and ketones are prepared by controlled oxidation of primary and secondary alcohols respectively using pyridinium chlorochromate (PCC) as the oxidizing agent 🧪✨
PCC is a mild oxidizing agent that selectively converts the –OH group into a carbonyl group (>C=O) without further oxidation to carboxylic acids. Hence, it prevents over-oxidation ⚖️
👉 Primary alcohols → Aldehydes
👉 Secondary alcohols → Ketones
👉 Tertiary alcohols do not undergo oxidation ❌
PCC is a yellow-orange compound with the formula [C₅H₅NH]⁺[CrO₃Cl]⁻ and is milder than chromic acid, making it ideal for controlled oxidation 🔶
🧪 Equations:
⚡ General Equation of Oxidation of Primary Alcohol to Aldehyde: RCH₂OH —PCC→ RCHO + H₂O
⚡ Example of Equation of ethyl alcohol (1°) to ethanal: CH₃CH₂OH —PCC→ CH₃CHO
⚡ General Equation of Oxidation of Secondary Alcohol to Ketone: R₂CHOH —PCC→ R₂CO + H₂O
⚡ Example of Equation of isopropyl alcohol (2°) to acetone: CH₃CHOHCH₃ —PCC→ CH₃COCH₃ + H₂O
Q6. Give equations for the reactions of propanone with the following reagents?
(a) Reaction with Hydrazine (NH₂NH₂)
👉 Formation of hydrazone
CH₃COCH₃ + NH₂NH₂ ⟶ CH₃C(=NNH₂)CH₃ or (CH₃)₂C=NNH₂ + H₂O 🧪 Condensation reaction
(b) Reaction with Hydroxylamine (NH₂OH)
👉 Formation of oxime
CH₃COCH₃ + NH₂OH ⟶ CH₃C(=NOH)CH₃ or (CH₃)₂C=NOH + H₂O ✨ Oxime formation
(c) Reaction with Hydrogen cyanide (HCN)
👉 Formation of cyanohydrin
CH₃COCH₃ + HCN ⟶ (CH₃)₂C(OH)CN 🎯 Nucleophilic addition reaction
Q7. How would you obtain carbonyl compounds from oxidation of alcohols and hydration of alkynes?
(A) Preparation of Carbonyl Compounds from Primary and Secondary Alcohols – Controlled Oxidation
🧪 Reagent: Pyridinium chlorochromate (PCC), a mild oxidant converts the –OH group into a carbonyl group (>C=O)
🧬 Formula: [C₅H₅NH]⁺[CrO₃Cl]⁻
⚙️ Function: ideal for controlled oxidation 🔶 PCC prevents over-oxidation of aldehydes to carboxylic acids ⚖️
👉 Primary alcohols → Aldehydes
👉 Secondary alcohols → Ketones
👉 Tertiary alcohols do not undergo oxidation ❌
🧪 Equations:
(i) General Reaction of Oxidation of Primary Alcohol to Aldehyde: RCH₂OH —PCC→ RCHO + H₂O
Example: CH₃CH₂OH —PCC→ CH₃CHO
(ii) General Reaction of Oxidation of Secondary Alcohol to Ketone: R₂CHOH —PCC→ R₂CO + H₂O
Example: CH₃CHOHCH₃ —PCC→ CH₃COCH₃ + H₂O
📌 Summary:
Controlled oxidation of alcohols → selective aldehydes/ketones
(B) Preparation of Carbonyl Compounds by Hydration of Alkynes
Alkynes react with water (H₂O) in the presence of acid and HgSO₄ catalyst → enol → tautomerizes to carbonyl compounds (acid-catalyzed hydration) ⚡💧
👉 Ethyne → Aldehydes
👉 Internal alkynes → Ketones
🧪 General Equation: RC≡CH + H₂O —[H₂SO₄/HgSO₄]→ R–C(OH)=CH₂ (enol) — tautomerization → RCOCH₃
📌 Example (Ethyne): HC≡CH + H₂O —[H₂SO₄/HgSO₄]→ CH₂=CH–OH (enol) — tautomerization → CH₃CHO
📌 Example (Propyne): CH₃C≡CH + H₂O —[H₂SO₄/HgSO₄]→ CH₃C(OH)=CH₂ (enol) — tautomerization → CH₃COCH₃
✨ Summary:
Acid-catalyzed hydration of alkynes → carbonyl compounds via enol → keto tautomerization
Q8. Complete and balance the following equations:
1️⃣ Addition of HCN to acetaldehyde (CH₃CHO) → cyanohydrin
CH₃CHO + HCN → CH₃CH(OH)CN ✅
2️⃣ Oxidation of formaldehyde (HCHO) → formic acid
HCHO + [O] → HCOOH ✅
3️⃣ Formation of phenylhydrazone from acetone (CH₃COCH₃) and phenylhydrazine (C₆H₅NHNH₂)
CH₃COCH₃ + C₆H₅NHNH₂ → CH₃C(=NNHC₆H₅)CH₃ or (CH₃)₂C=NNHC₆H₅ + H₂O ✅
4️⃣ Formation of oxime from formaldehyde (HCHO) and hydroxylamine (NH₂OH)
HCHO + NH₂OH → HCH=NOH + H₂O ✅
5️⃣ Friedel–Crafts acylation (benzene + acetyl chloride)
C₆H₆ + CH₃COCl → C₆H₅COCH₃ + HCl ✅
✏️ Smart Answers of Descriptive Questions of Aldehydes and ketones✏️
Q1. What are aldehydes and ketones? Describe the structure and type of hybridization in them.
⭐ Aldehydes
✨ Carbonyl Compounds: Aldehydes and ketones both are known as Carbonyl Compounds as both contain carbonyl group
✨ Definition: They are carbonyl compounds containing a –CHO (formyl) group at the terminal carbon of the chain.
🔹 Carbonyl carbon is bonded to one hydrogen and one alkyl group (formaldehyde: both H).
✨ General formula: CₙH₂ₙO
✨ Functional group: –CHO (aldehydic or aldo or formyl group) 🧪
⭐ Ketones
✨ Definition: Carbonyl compounds containing a C=O group within the carbon chain (not terminal).
🔹 Carbonyl carbon is bonded to two alkyl groups.
✨ General formula: CₙH₂ₙO
✨ Functional group: –COR (ketonic or keto group) 🧪
⭐ Structure and Hybridization of Carbonyl Group (C=O)
➡️ Carbonyl Hybridization: Carbon in C=O is sp²-hybridized
➡️ Types of overlapping:
⚡ Sigma bonds with oxygen and other atoms
⚡ Pi bond with oxygen using unhybridized p-orbital
➡️ Geometry: Trigonal planar, bond angles ≈ 120°
➡️ Planarity makes carbonyl carbon electrophilic, explaining its reactivity ⚡
⭐ Summary Table:
➡️ Aldehyde: –CHO functional group | Carbonyl at terminal position | Carbonyl carbon sp² hybridized | Trigonal planar geometry
➡️ Ketone: –C(=O)R functional group | Carbonyl at internal position | Carbonyl carbon sp² hybridized | Trigonal planar geometry
Q2. Explain the acid-catalyzed and base-catalyzed nucleophilic addition reactions in aldehydes and ketones.
✨ Nucleophilic Addition Reactions Overview
Nucleophilic addition reactions occur via two mechanisms:
➡️ Base-catalyzed addition (neutral or basic conditions)
➡️ Acid-catalyzed addition (acidic conditions)
🧪 Acid-Catalyzed Nucleophilic Addition Reactions of Carbonyl Compounds
➡️ An acid catalyst is used in a case where a carbonyl compound reacts with weak nucleophile for addition.
⚙️ Mechanism:
➡️ Acid catalyst (H⁺) protonates the carbonyl group, making the carbonyl carbon more electrophilic, forming a protonated carbonyl group.
➡️ This increases the carbonyl carbon’s attractiveness to weak nucleophiles.
➡️ The weak nucleophile attacks the carbonyl carbon, leading to the addition product.
➡️ Condensation-Elimination or Addition-Elimination Reaction
➡️ Definition: In Condensation-Elimination or Addition-Elimination Reaction C=O is converted into C=N.
➡️ Examples: Addition of ammonia (NH₃), hydrazine (NH₂NH₂), hydrazine derivatives, hydroxylamine (NH₂OH), alcohols, or amines to carbonyl groups.
⚙️ Mechanism Steps:
Step 1: Nucleophile attacks carbonyl → unstable intermediate.
Step 2: Loss of water molecule → stable addition product (like C=N).
🔍 Reaction Examples
📌 (i) Hydrazine Addition (acid-catalyzed reaction):
RCHO + NH₂NH₂ —Acid/H⁺ → H₂O + RCH=N–NH₂
RCOR + NH₂NH₂ —Acid/H⁺ → H₂O + R₂C=N–NH₂
OR R–C(=O)–R′ + H₂N–NH₂ —Acid/H⁺ → H₂O + R–C(=N–NH₂)–R′
📌 (ii) Hydroxylamine Addition (acid-catalyzed reaction):
RCHO + NH₂OH —Acid/H⁺ → H₂O + RCH=N–OH (Aldo Oxime)
RCOR + NH₂OH —Acid/H⁺ → H₂O + R₂C=N–OH (Keto Oxime)
OR R–C(=O)–R′ + H₂N–OH —Acid/H⁺ → H₂O + R–C(=N–OH)–R′
Examples:
HCHO + H₂N–OH —Acid/H⁺ → H₂O + H₂C=N–OH
CH₃CHO + H₂N–OH —Acid/H⁺ → H₂O + CH₃–CH=N–OH
CH₃COCH₃ + H₂N–OH —Acid/H⁺ → H₂O + (CH₃)₂C=N–OH
📌 Reduction Example (Wolf-Kishner Reaction):
➡️ Aldehyde/ketone reduced to alkane by hydrazine (NH₂NH₂) and potassium hydroxide (KOH).
➡️ Product: Alkane
➡️ Reagents: Hydrazine (H₂NNH₂), strong base (KOH), heat
➡️ General Equation for Aldehydes: R–C(=O)–H + H₂NNH₂ —KOH/200°C → R–CH₂–H + N₂ ↑ + H₂O
➡️ General Equation for Ketones: R–C(=O)–R′ + H₂NNH₂ —KOH/200°C → R–CH₂–R′ + N₂ ↑ + H₂O
Example: CH₃COCH₃ + H₂NNH₂ —KOH/200°C → CH₃CH₂CH₃ + N₂ ↑ + H₂O
🔧 Base-Catalyzed Nucleophilic Addition Reactions
➡️ Addition of strong nucleophilic reagents on aldehydes and ketones is catalyzed by a base.
⚙️ Steps of Mechanism:
➡️ A strong base generates a nucleophile (OH⁻ or R⁻) by deprotonating the nucleophile.
➡️ The nucleophile then attacks the carbonyl carbon.
➡️ The C=O bond pi electrons shift towards oxygen atom, creating a tetrahedral intermediate (alkoxide).
➡️ The intermediate captures a proton or electrophile to form the final addition product.
🔍 Examples of Base-Catalyzed Reactions:
📌 Addition of HCN (Base-catalyzed):
RCHO + HCN → RCH(OH)CN
RCOR + HCN → R₂CH(OH)CN
📌 Addition of Grignard Reagent (Base-catalyzed):
RMgX attacks aldehydes/ketones → alkoxide intermediate → hydrolysis → alcohols.
Outcome:
Formaldehyde → Primary alcohol
Higher aldehydes → Secondary alcohol
Ketones → Tertiary alcohol
⚙️ Mechanism:
🧪 Grignard reagent (RMgX) acts as a strong nucleophile (R⁻)
⚡ Nucleophilic attack on C=O → alkoxide intermediate ➕
💧 Protonation → alcohol product 🍺
General Equations:
HCHO + RMgX → RCH(OMgX)H → RCH(OH)H
RCHO + RMgX → RCH(OMgX)R′ → RCH(OH)R′
RCOR + RMgX → RCH(OMgX)R′ → RCH(OH)R′
⚗️ Key Takeaways:
⚗️ Acid-catalyzed: weak nucleophiles (NH₃, NH₂NH₂, NH₂OH) → C=N bond formation 🧬
⚗️ Base-catalyzed: strong nucleophiles (HCN, RMgX) → cyanohydrins / alcohols 🧪🍺
📌 Notes:
⚡ Acid-catalyzed reactions make the carbonyl carbon more electrophilic.
⚡ Base-catalyzed reactions form a strong nucleophile that attacks the carbonyl carbon.
⚡ Grignard reagents produce alcohols, with different primary, secondary, or tertiary alcohols based on the carbonyl compound.
Q3. Describe how aldehydes are distinguished from ketones by the following laboratory tests:
🥈 (i) Tollen’s Test / Silver Mirror Test Using Tollen’s reagent 🪞
🧪 Reaction with Aldehydes:
➡️ Aldehydes (e.g., formaldehyde, acetaldehyde) react with Tollen’s reagent (ammoniacal silver nitrate) on warming to reduce Ag⁺ ions to metallic silver. This is known as Tollen’s Test.
➡️ This forms a silver mirror on the surface of the test tube.
➡️ Aldehyde (oxidized) → Carboxylic acid (which forms ammonium salt with NH₄OH, ammonium carboxylate)
➡️ Tollen’s reagent (reduced) → Silver mirror (metallic silver)
🧪 Reaction with Ketones:
➡️ Ketones (e.g., acetone) do not react with Tollen’s reagent and do not form the silver mirror.
⚗️ General Chemical Equation (Tollen’s Test):
RCHO + 2[Ag(NH₃)₂]OH — Warm → RCOONH₄ + 2Ag (silver mirror) + 3NH₃ + H₂O
📌 Examples:
HCHO + 2[Ag(NH₃)₂]OH — Warm → HCOONH₄ + 2Ag ↓ + 3NH₃ + H₂O
CH₃CHO + 2[Ag(NH₃)₂]OH — Warm → CH₃COONH₄ + 2Ag ↓ + 3NH₃ + H₂O
🔴 (ii) Fehling’s Test Using Fehling’s Solution 🧪
🧪 Reaction with Aldehydes:
➡️ Aldehydes (e.g., acetaldehyde) react with Fehling’s solution (which contains Cu²⁺ ions) on warming to reduce Cu²⁺ to Cu₂O (cuprous oxide), forming an orange-red precipitate.
➡️ Fehling’s solution: an alkaline solution of a cupric ion complexed with sodium potassium tartrate.
➡️ Formula is complex [Cu(tartarate)₂]²⁻ but represented as Cu(OH)₂.
📌 CuSO₄ + 2NaOH — Tartaric acid → Na₂SO₄ + Cu(OH)₂
➡️ Aldehyde (oxidized) → Carboxylic acid (forms sodium carboxylate with NaOH)
➡️ Fehling’s solution (reduced) → Cu₂O (orange-red ppt.)
🧪 Reaction with Ketones:
➡️ Ketones (e.g., acetone) do not react with Fehling’s solution, and no precipitate is formed.
⚗️ Chemical Equation (Fehling’s Test):
📌 RCHO + Cu(OH)₂ — Warm → RCOOH + Cu₂O (orange-red ppt.)
📌 Summary:
🥈 Tollen’s Test 🪞:
🧪 Aldehyde → reduces silver ions to metallic silver → Silver mirror 🪞
❌ Ketone → no reaction, no silver mirror
🔴 Fehling’s Test ⚗️:
🧪 Aldehyde → reduces Cu²⁺ to Cu₂O → Orange-red precipitate
❌ Ketone → no reaction, no precipitate
📌 Key Takeaways:
➡️ Aldehydes are oxidized in both tests (forming carboxylic acids).
➡️ Ketones are not oxidized in either test, showing no reaction or no precipitate.
Q4. Write the equation and give the name of major product of the following chemical process:
🔥 (i) Oxidation of Acetone with Acidified K₂Cr₂O₇ into Acetic Acid 🔴
⚗️ Reagent: Acidified Potassium Dichromate (K₂Cr₂O₇) acts as an oxidizing agent ⚡
⚡ Reaction: Acetone (CH₃COCH₃) is oxidized to a carboxylic acid (acetic acid, CH₃COOH). 🍶
🏆 Major Product: Acetic acid (CH₃COOH) 🍶
🧪 Equation: CH₃COCH₃ + 4[O] —K₂Cr₂O₇/H₂SO₄→ CH₃COOH + CO₂ + H₂O
🔥 (ii) Reduction of Acetaldehyde with NaBH₄ into Ethyl Alcohol 🔵
⚗️ Reagent: Sodium Borohydride (NaBH₄) as a reducing agent
⚡ Reaction: Acetaldehyde (CH₃CHO) is reduced to ethanol (CH₃CH₂OH).
🏆 Major Product: Ethanol (CH₃CH₂OH)
🧪 Equation: CH₃CHO + H₂ —NaBH₄→ CH₃CH₂OH
🔥 (iii) Hydration of Ethyne (Acetylene) into Acetaldehyde 💧
⚗️ Reagent: Water (H₂O), H₂SO₄ (acid catalyst), and HgSO₄ (mercuric sulfate)
⚡ Reaction: Ethyne (C₂H₂) reacts with water to form acetaldehyde (CH₃CHO) under acidic conditions.
🏆 Major Product: Acetaldehyde (CH₃CHO)
🧪 Equation: HC≡CH + H₂O —H₂SO₄/HgSO₄ (100°C)→ H₂C=CHOH —Tautomerization→ CH₃CHO
🔥 (iv) Acylation of Benzene in the Presence of AlCl₃ into Acetophenone ⚙️
⚗️ Reagent: Acetyl chloride (CH₃COCl) and AlCl₃ (Lewis acid catalyst)
⚡ Reaction: Benzene (C₆H₆) undergoes Friedel–Crafts acylation to form acetophenone (C₆H₅COCH₃).
🏆 Major Product: Acetophenone (C₆H₅COCH₃)
🧪 Equation: C₆H₆ + CH₃COCl —AlCl₃→ C₆H₅COCH₃ + HCl
📌 Summary Table:
| Reaction | Reagent | Equation | Major Product |
| Oxidation of Acetone | Acidified K₂Cr₂O₇ | CH₃COCH₃ + 4[O] —K₂Cr₂O₇/H₂SO₄→ CH₃COOH + CO₂ + H₂O | Acetic acid (CH₃COOH) |
| Reduction of Acetaldehyde | NaBH₄ | CH₃CHO + H₂ —NaBH₄→ CH₃CH₂OH | Ethanol (CH₃CH₂OH) |
| Hydration of Ethyne | H₂O, H₂SO₄, HgSO₄ | HC≡CH + H₂O —H₂SO₄/HgSO₄→ H₂C=CHOH —Tautomerization→ CH₃CHO | Acetaldehyde (CH₃CHO) |
| Acylation of Benzene | CH₃COCl, AlCl₃ | C₆H₆ + CH₃COCl —AlCl₃→ C₆H₅COCH₃ + HCl | Acetophenone (C₆H₅COCH₃) |
Q5. Give four differences between aldehydes and ketones.
🧬 Aldehydes 🧪 vs Ketones ⚗️
| Aldehydes (–CHO) 🧪 | Ketones (–CO–) ⚗️ |
| Contain aldehydic group (–CHO) 🔹 | Contain keto group (–COR or –CO–) 🔹 |
| Carbonyl carbon attached to at least one H atom 🧬 | Carbonyl carbon attached to two alkyl/aryl groups 🔗 |
| Functional group always at the end of the chain ➡️ | Functional group always in the middle of the chain ↔️ |
| IUPAC suffix: –al 🏷️ | IUPAC suffix: –one 🏷️ |
| More reactive (less steric hindrance) ⚡ | Less reactive (more steric hindrance) |
| On reduction → primary alcohol 🍶 | On reduction → secondary alcohol 🍷 |
| Easily oxidized to acids 🔥 | Resistant to oxidation (needs strong oxidants) |
| Positive Fehling’s, Tollen’s & Benedict’s tests ✅ | Negative results ❌ |
| Silver mirror in Tollen’s test ✨ | No silver mirror formed |
| Pink colour in Schiff’s test 🌸 | No colour change |
| Lower boiling point 🌡️ | Higher boiling point |
| Readily undergo polymerization 🔁 | Do not polymerize 🚫 |
📌 Summary Table:
| Feature | Aldehydes (–CHO) | Ketones (–CO–) |
| Functional Group | –CHO (formyl group) | –COR (keto group) |
| Attachment | Carbonyl carbon attached to ≥1 H atom | Carbonyl carbon attached to two alkyl/aryl groups |
| Position in Chain | Always terminal | Always internal |
| IUPAC Suffix | –al | –one |
| Reactivity | More reactive (less steric hindrance) | Less reactive (more steric hindrance) |
| Reduction | Gives primary alcohol | Gives secondary alcohol |
| Oxidation | Easily oxidized to acids | Resistant, needs strong oxidants |
| Tests | Positive Fehling’s, Tollen’s, Benedict’s | Negative results |
| Tollen’s Test | Silver mirror formed | No silver mirror |
| Schiff’s Test | Pink colour | No colour change |
| Boiling Point | Lower | Higher |
| Polymerization | Readily undergo polymerization | Do not polymerize |
Q6. Write the equation for the nucleophilic addition reaction if formaldehyde treated with:
🧪 i. Formaldehyde + Hydrogen cyanide (HCN) → Cyanohydrin formation
🧬 Reaction equation: HCHO + HCN → HO–CH₂–CN ➡ Product: Cyanohydrin 🟢
🧪 ii. Formaldehyde + Primary alcohol (e.g. methanol) → Hemiacetal formation (acid-catalysed)
🧬 Reaction equation: HCHO + CH₃OH → HO–CH₂–OCH₃ ➡ Product: Hemiacetal 🟢
🧪 iii. Formaldehyde + Methyl magnesium bromide (CH₃MgBr) → Grignard reaction followed by hydrolysis
🧬 Reaction equation: HCHO + CH₃MgBr → CH₃CH₂OMgBr + H₂O → CH₃CH₂OH ➡ Product: Ethanol (primary alcohol) 🟢
🧪 iv. Formaldehyde + Ammonia (NH₃) → Formation of imine/Schiff base (via carbinolamine)
🧬 Reaction equation: HCHO + NH₃ → CH₂=NH + H₂O 🟢
Q7. Explain the factors that influence the reactivity of carbonyl compounds towards nucleophilic addition reaction
1️⃣ Steric Effect or Steric Hindrance (Crowdedness around Electrophilic C=O) 🚧
➡️ Reactivity decreases when bulky groups block the nucleophile’s approach (steric effects).
➡️ Less crowded carbonyl carbon → faster nucleophilic attack.
➡️ Aldehydes > Ketones in reactivity due to lower steric hindrance.
➡️ Ketones react slower because two alkyl groups create steric bulk.
📌 Order of reactivity: HCHO > RCHO > R₂CO
2️⃣ Amount of Positive Charge on Carbonyl Carbon (Electronic Effect) ⚡
➡️ Nucleophiles attack the electrophilic carbonyl carbon (Cδ⁺).
➡️ Greater positive charge on carbonyl carbon → higher reactivity.
➡️ Electron-withdrawing groups (–I) increase electrophilicity → higher reactivity.
➡️ Electron-donating alkyl groups (+I) decrease electrophilicity → lesser reactivity.
👉 Aldehydes are more reactive than ketones because ketones have two +I alkyl groups, which reduce the positive charge on C=O carbon.
🍋 Acid-Catalysed Nucleophilic Addition Reactions
➡️ Used when weak nucleophiles (H₂O, ROH) are involved.
➡️ Acid protonates oxygen → protonated carbonyl group → increasing electrophilicity of carbonyl carbon.
Equation:
C=O + H⁺ → C=OH⁺
C=OH⁺ + Nu⁻ → Addition product
✔ Protonation makes carbonyl carbon more susceptible to nucleophilic attack.
🧪 Base-Catalysed Nucleophilic Addition Reaction
➡️ Used with strong nucleophiles (CN⁻, RMgX, OH⁻).
➡️ Base generates nucleophile with the reagent which attacks carbonyl carbon.
➡️ Formation of tetrahedral alkoxide intermediate that captures a proton or the electrophile to give the addition product.
Equation:
C=O + Nu⁻ → C–O⁻ (alkoxide)
C–O⁻ + H⁺ → Addition product
✔ Reaction proceeds faster due to high nucleophilicity.
🔑 One-Line Summary for Exams 📝
Reactivity of carbonyl compounds toward nucleophilic addition depends on steric hindrance, electrophilicity of carbonyl carbon, and acid or base catalysis.
Q8. Describe oxidation of carbonyl compounds.
🔥 Oxidation of Carbonyl Compounds into Carboxylic Acids
➡️ Carbonyl compounds undergo oxidation with loss of two hydrogen atoms.
➡️ Oxidation generally converts carbonyl compounds into carboxylic acids.
🧪 1. Oxidation of Aldehydes into Corresponding Carboxylic Acids ➡️🧴 (Easy oxidation)
➡️ Aldehydes are readily oxidized due to presence of active hydrogen at –CHO group.
➡️ Oxidation does not break C–C bond.
➡️ Both mild and strong oxidizing agents can be used.
⚗️ Common oxidizing agents: Chromic acid (H₂CrO₄), Tollen’s reagent, Fehling’s solution, Benedict’s reagent.
🏆 Product: Corresponding carboxylic acid with same number of carbon atoms.
Equation: R–CHO + [O] → R–COOH
⚗️ 2. Oxidation of Ketones into Lower Carboxylic Acids ➡️🔥 (Difficult oxidation)
➡️ Ketones are resistant to oxidation.
➡️ Oxidation requires breaking of C–C sigma bond, hence reaction is slow.
➡️ Only strong oxidizing agents can oxidize ketones.
⚗️ Oxidizing agents used: Acidified potassium dichromate (K₂Cr₂O₇ / H₂SO₄), Acidified potassium permanganate (KMnO₄).
🏆 Product: Lower carboxylic acids (one carbon atom is lost as CO₂).
Equation: R–CO–CH₃ + [O] → R–COOH + CO₂ + H₂O
🔑 Key Differences (One-Look Memory) 🧠
➡️ Aldehydes: easily oxidized ✅
➡️ Ketones: difficult to oxidize ❌
➡️ Aldehydes give same-carbon acids.
➡️ Ketones give lower acids + CO₂.
📝 One-Line Exam Summary
Aldehydes are easily oxidized to corresponding carboxylic acids by mild oxidizing agents, whereas ketones require strong oxidizing agents and undergo oxidative cleavage to form lower carboxylic acids.
💥 جونؔ ایلیا ✨🎯💔
🌹 تم سے مل کر بہت خوشی ہو کیا
🔥 مجھ کو یکسر بھلا چکی ہو کیا
😔 مجھ سے مل کر اداس بھی ہو کیا
🤔 سوچتی ہو تو سوچتی ہو کیا
🌷 اب بھی تم میری زندگی ہو کیا
🕊️ آخری بار مل رہی ہو کیا
🌞 تو بہت تیز روشنی ہو کیا
🚶 تم بہت دور جا چکی ہو کیا
🔥 شمع امید بجھ گئی ہو کیا
💧 بان تم اب بھی بہہ رہی ہو کیا
▶ Watch Video on YouTube
Click the preview below to open in new window

▶ Click image to watch on YouTube

💥 جونؔ ایلیا ✨🎯💔
اس کے بغیر اس کی تمنا کیے بغیر
دیوار تک نہیں گری پردا کیے بغیر
بے حد عزیز ہے مجھے اچھا کیے بغیر
صبح ازل سے کوئی تقاضا کیے بغیر
وہ مجھ کو چاہئے کوئی سودا کیے بغیر
وعدہ ہمیں قبول ہے ایفا کیے بغیر
میں ہوں نہ قتل کوئی تماشا کیے بغیر
تم چھوڑیو نہ شہر کو صحرا کیے بغیر
آرائش نظر تری پروا کیے بغیر
سب کچھ ہے خوش گوار گوارا کیے بغیر
ہم گریہ کن ازل کے ہیں گریہ کیے بغیر
تاریخ کے حرام سے توبہ کیے بغیر
شیعہ بنا دیا ہمیں شیعہ کیے بغیر
رخصت کرو مجھے کوئی وعدہ کیے بغیر
Practice Model Test Questions for Class 12 Chemistry, Chapter 8 – Aldehydes and Ketones (Test #9). Includes MCQs, short-answer, and numerical questions for board exams, MDCAT, and ECAT preparation.
Welcome to Learn Chemistry by Inam Jazbi! This post contains Model Test Questions for Class 12 Chemistry, Chapter 8 – Aldehydes and Ketones (Test #9).
These questions include MCQs, short-answer questions, and numericals, designed to help students practice efficiently and revise key concepts. Ideal for board exams and competitive tests like MDCAT and ECAT, this test covers all important points from the chapter.
🧠 Related Posts:
https://learnchemistrybyinamjazbi.blogspot.com/2024/11/iupac-nomenclature-of-organic-compounds.html
https://learnchemistrybyinamjazbi.blogspot.com/2025/11/class-12-chemistry-notes-benzene-and.html
https://learnchemistrybyinamjazbi.blogspot.com/2025/11/class-12-chemistry-notes-alkynes.html
https://learnchemistrybyinamjazbi.blogspot.com/2025/11/xii-chemistry-notes-ozonolysis-or.html
https://learnchemistrybyinamjazbi.blogspot.com/2025/02/xii-chemistry-model-test-questions.html
https://learnchemistrybyinamjazbi.blogspot.com/2024/10/alkyl-group.html
https://learnchemistrybyinamjazbi.blogspot.com/2024/09/diagonal-relationship-of-representative.html
https://learnchemistrybyinamjazbi.blogspot.com/2024/08/general-group-trends-of-representative.html
https://learnchemistrybyinamjazbi.blogspot.com/2024/08/flame-test-for-s-block-elements.html
https://learnchemistrybyinamjazbi.blogspot.com/2024/08/xii-chemistry-model-test-questions-xii.html
https://learnchemistrybyinamjazbi.blogspot.com/2024/08/ixxii-valency.html
https://learnchemistrybyinamjazbi.blogspot.com/2024/08/xii-completes-chemistry-notes-nationals.html
https://learnchemistrybyinamjazbi.blogspot.com/2024/03/xii-chemistry-model-test-questions-test.html