🧪 Class 12 Chemistry – Chapter 7: Alcohols & Ethers ✨
Preparing for Class 12 Chemistry exams and worried about Chapter 7: Alcohols and Ethers? 😟 You’re in the right place! ✅
In this blog, Inam Jazbi’s smart, board-oriented model test questions are carefully designed to sharpen your concepts, boost your confidence 💪, and help you score maximum marks 📈.
These questions strictly follow the latest exam pattern, covering important numericals, MCQs, short and long questions, with special focus on the areas examiners love to repeat 🔁.
If you want easy understanding + high scores 🏆, this guide is a must-read before your chemistry paper! 📘🔥
✏️ Model Test Questions XII Chemistry Chapter # 7………… Alcohols and Ethers ✏️
✏️ Short Questions of Alcohols and Ethers ✏️
(i) Reduction of acetic acid with LiAlH₄
(ii) Hydration of ethene with hot concentrated H₂SO₄
(iii) Oxidation of ethanol with acidified dichromate
(iv) Hydrolysis of diazonium salt
(v) Condensation of alcohols into ether
(i) Boiling point of ether is less than alcohol
(ii) Alcohols are soluble in water
(iii) Ethanol is liquid but ethyl chloride is gas at room temperature
✏️ Descriptive Questions of Alcohols and Ethers ✏️
(i) Ethyl alcohol to diethyl ether
(ii) Phenol into benzoquinone
(iii) Ethyl bromide to ethanol
(iv) 2°-alcohol to carboxylic acid
(v) Ethanol into acetic acid
(vi) Ethyl alcohol into ethyne
(vii) Ethene into ethanol
(i) Solubility in water
(ii) Boiling point
(iii) Acidic character
(i) Hot and concentrated nitric acid
(ii) Concentrated sulphuric acid at 100°C
(iii) Bromine water
(iv) Sodium metal
✏️ Text Book Multiple Choice Questions on Chapter 7 ✏️
Reason: The carbon is part of an aromatic ring, therefore it is sp²-hybridized.
Reason: Pyrogallol contains three –OH groups, hence it is trihydric.
Reason: PCl₃ replaces the –OH group of alcohol with –Cl.
Reason: Straight-chain alcohols have stronger intermolecular forces.
Reason: At 170°C, dehydration of ethanol leads to elimination forming ethene.
Reason: Lucas reagent consists of anhydrous ZnCl₂ dissolved in concentrated HCl.
Reason: Periodic acid (HIO₄) cleaves vicinal diols by oxidative cleavage.
Reason: Ethyl chloride does not contain –OH or –NH group required for hydrogen bonding.
Reason: Secondary alcohols are first oxidized to ketones during oxidation.
Reason: Diethyl ether has been used as a general anaesthetic.
✏️ Multiple Choice Questions on Alcohols and Ethers from Past Papers✏️
Reason: Ethylene glycol (HO–CH₂–CH₂–OH) has two hydroxyl groups, making it dihydric.
Reason: Glycerol (HO–CH₂–CHOH–CH₂–OH) has three –OH groups, hence trihydric.
Reason: Alcohols show chain, position, and functional isomerism.
Reason: Ethanol and CH₃OCH₃ are functional isomers (alcohol ↔ ether).
Reason: Secondary alcohols have –OH attached to a carbon which is bonded to two other carbons.
Reason: Methanol is called wood spirit because it was originally obtained by distillation of wood.
Reason: Methyl alcohol (CH₃OH) is commonly called carbinol.
Reason: Ethanol oxidized by Acetobacter bacteria produces acetic acid (vinegar).
Reason: Chronic alcohol abuse can damage the liver, causing cirrhosis.
Reason: Oxidation of primary alcohols with nascent oxygen produces aldehydes (alkanals).
Reason: Secondary alcohols are oxidized to ketones by oxidizing agents.
Reason: Acid-catalyzed reaction of carboxylic acids with alcohols forms esters.
Reason: Lucas test (ZnCl₂ + HCl) differentiates primary, secondary, and tertiary alcohols.
Reason: Triesters of glycerol and fatty acids are called triglycerides, triacylglycerols, and fats/oils.
Reason: Oxidation of alcohols involves breaking O-H and the hydrogen from C-H.
Reason: Replacement of –OH with halogen (cleaving O-H) forms alkyl halides.
Reason: Cleavage of C-O bond of alcohols gives alkyl halides.
Reason: Cleaving O-H bond cannot form simple alkanes.
Reason: Oxidation cleaves C-O bond to form aldehydes or ketones.
Reason: Oxidation involves breaking C-H and C-O bonds, forming aldehydes/ketones.
Reason: Alcohols do not undergo addition reactions; they mainly undergo substitution, condensation, and elimination.
Reason: Primary carbocation CH₃⁺ is least stable due to no alkyl group stabilization.
Reason: Alcohols lose water (–OH and H) in the presence of acid to form alkenes.
Reason: Alcoholic KOH causes elimination (dehydrohalogenation) to form an alkene.
Reason: Alcohol reacts with metals to form alkoxide salts (RO⁻M⁺).
Reason: Ethanol oxidized by Acetobacter bacteria produces acetic acid (vinegar).
Reason: Alcoholic KOH promotes elimination to give the 1-alkene.
Reason: Long hydrocarbon chains in alcohols reduce polarity, decreasing water solubility.
Reason: Methanol is commonly called wood spirit, wood naphtha, and carbinol.
Reason: Primary alcohol oxidizes first to an aldehyde before further oxidation to carboxylic acid.
Reason: Alcohols show both acidic (–OH can donate H⁺) and basic (lone pair on O) behavior, demonstrating amphoteric nature.
✏️ Smart Answers of Model Test Questions XII Chemistry Test # 8 Chapter # 7………… Alcohols and Ethers ✏️
✏️ Answers of Short Questions of Alcohols and Ethers✏️
Q1. What are alcohols? How alcohols are classified? Define primary, secondary & tertiary alcohols. Write the structures & names of primary, secondary & tertiary alcohols of molecular formula C₅H₁₁OH (8 isomers).
Answer
🧪 Alcohols – Summary & Classification
✴️ Definition: Alcohols are aliphatic organic compounds containing one or more hydroxyl (–OH) groups attached to a saturated carbon atom.
👉 Derivatives of alkanes (replace H with –OH) or derivatives of water (replace H with alkyl group).
✴️ Monohydric Alcohols (Alkanols)
📌 Definition: Saturated aliphatic alcohols with one –OH group.
📌 General formula: CₙH₂ₙ₊₁OH or CₙH₂ₙ₊₂O
📌 Type formula: R–OH
🧪 Examples:
CH₃OH → Methanol
CH₃CH₂OH → Ethanol
CH₃CH₂CH₂OH → 1‑Propanol
✴️ Classification Based on Number of –OH Groups
🟢 Monohydric ➡️ One –OH group
🧪 Example: CH₃OH, C₂H₅OH, (CH₃)₂CHOH
🟢 Dihydric ➡️ Two –OH groups
🧪 Example: HO–CH₂–CH₂–OH (Ethylene glycol)
🟢 Trihydric ➡️ Three –OH groups
🧪 Example: HO–CH₂–CH(OH)–CH₂–OH (Glycerol)
🟢 Polyhydric ➡️ Four or more –OH groups
🧪 Example: Sorbitol, Mannitol
✴️ Classification Based on Nature of α‑Carbon
🟠 1° (Primary) Alcohols
➡️ –OH on carbon attached to one other C
🧪 Group: –CH₂–OH
🧪 Example: CH₃CH₂OH (Ethanol), CH₃CH₂CH₂OH (1‑Propanol)
🟡 2° (Secondary) Alcohols
➡️ –OH on carbon attached to two other C
🧪 Group: >CH–OH
🧪 Example: CH₃–CHOH–CH₃ (2‑Propanol)
🔴 3° (Tertiary) Alcohols
➡️ –OH on carbon attached to three other C
🧪 Group: ⪫C–OH
🧪 Example: (CH₃)₃COH (tert‑Butyl alcohol)
Draw possible isomers of C₅H₁₁OH
Answer
🟢 Primary (1°) Alcohols – 4 isomers
(–OH on a carbon attached to only one other carbon)
1️⃣ Pentan‑1‑ol → CH₃–CH₂–CH₂–CH₂–CH₂–OH
2️⃣ 2‑Methylbutan‑1‑ol → CH₃–CH(CH₃)–CH₂–CH₂–OH
3️⃣ 3‑Methylbutan‑1‑ol (Isoamyl alcohol) → CH₃–CH₂–CH(CH₃)–CH₂–OH
4️⃣ 2,2‑Dimethylpropan‑1‑ol (Neopentyl alcohol) → (CH₃)₃CCH₂OH
🟡 Secondary (2°) Alcohols – 3 isomers
(–OH on a carbon attached to two other carbon atoms)
5️⃣ Pentan‑2‑ol → CH₃–CH(OH)–CH₂–CH₂–CH₃
6️⃣ Pentan‑3‑ol → CH₃–CH₂–CH(OH)–CH₂–CH₃
7️⃣ 3‑Methylbutan‑2‑ol → CH₃–CH(OH)–CH(CH₃)–CH₃
🔴 Tertiary (3°) Alcohol – 1 isomer
(–OH on a carbon attached to three other carbon atoms)
8️⃣ 2‑Methyl‑2‑butanol (tert‑Amyl alcohol) → (CH₃)₂C(CH₂CH₃)OH
Q2. Give two examples of each of monohydric, dihydric and trihydric alcohols with their IUPAC names.
Answer
🧪 Examples of Alcohols (with IUPAC Names)
✴️ Monohydric Alcohols (Contain one –OH group)
1️⃣ Ethanol 🧬 Structure: CH₃–CH₂–OH 📘 IUPAC name: Ethanol
2️⃣ Propan‑1‑ol 🧬 Structure: CH₃–CH₂–CH₂–OH 📘 IUPAC name: Propan‑1‑ol
✴️ Dihydric Alcohols / Glycols (Contain two –OH groups)
1️⃣ Ethane‑1,2‑diol (Ethylene glycol) 🧬 Structure: HO–CH₂–CH₂–OH 📘 IUPAC name: Ethane‑1,2‑diol
2️⃣ Propane‑1,3‑diol 🧬 Structure: HO–CH₂–CH₂–CH₂–OH 📘 IUPAC name: Propane‑1,3‑diol
✴️ Trihydric Alcohols (Contain three –OH groups)
1️⃣ Propane‑1,2,3‑triol (Glycerol) 🧬 Structure: HO–CH₂–CH(OH)–CH₂–OH 📘 IUPAC name: Propane‑1,2,3‑triol
2️⃣ Butane‑1,2,4‑triol 🧬 Structure: HO–CH₂–CH(OH)–CH₂–CH₂–OH 📘 IUPAC name: Butane‑1,2,4‑triol
Q3. Write the equations for the following chemical processes:
(i) Reduction of acetic acid with LiAlH₄
(ii) Hydration of ethene with hot concentrated H₂SO₄
(iii) Oxidation of ethanol with acidified dichromate (Oxidation of alcohols)
(iv) Hydrolysis of diazonium salt
(v) Condensation of alcohols into ether
Answer
🧪 Equations of Chemical Processes
♻️ (i) Reduction of Acetic Acid with LiAlH₄ (reducing agent) into ethyl alcohol (1°‑alcohol)
⚖️Equation: CH₃COOH + 4[H] — (LiAlH₄/dry ether) → CH₃CH₂OH + 2H₂O (reduction)
♻️ (ii) Acid‑catalyzed Hydration of Ethene into ethanol with Hot Concentrated H₂SO₄ (E₁)
⚖️Equations: CH₂=CH₂ + H–OH —(Conc. H₂SO₄, hot/100°C)→ CH₃CH₂OH
OR
Ethene forms ethanol via ethyl hydrogen sulphate as intermediate.
⚖️Equations: CH₂=CH₂ + H₂SO₄ — (conc., hot) → CH₃CH₂OSO₃H + H₂O → CH₃CH₂OH + H₂SO₄
♻️ (iii) Oxidation of Ethanol with Acidified Dichromate first to aldehyde, then to acid
⚖️Equations: CH₃CH₂OH + [O] — (K₂Cr₂O₇ / H₂SO₄)/–H₂O → CH₃CHO + [O] — (K₂Cr₂O₇ / H₂SO₄)/–H₂O → CH₃COOH
♻️ (iv) Hydrolysis of Aryl Diazonium Salt by hot water into phenol
⚖️Equation: C₆H₅N₂⁺Cl⁻ + H₂O — Heat → C₆H₅OH + N₂↑ + HCl
♻️ (v) Condensation (intermolecular dehydration) of excess of Alcohols into Ether in acidic medium at 140°C
⚖️Equation: 2CH₃CH₂OH — (Conc. H₂SO₄, 140°C) → CH₃CH₂–O–CH₂CH₃ + H₂O
Q4. Explain the following scientific reason:
(i) Boiling point of ether is less than alcohol?
(ii) Alcohols are soluble in water.
(iii) Ethanol is liquid but ethyl chloride is gas at room temperature.
Answer
(i) Boiling point of ether is lower than alcohol 🔥
Ether does not form intermolecular hydrogen bonds, whereas alcohol does. Hence ether has a lower boiling point.
(ii) Alcohols are soluble in water 💧
Lower alcohols (up to three carbon atoms) form hydrogen bonds with water due to the presence of the polar –OH group.
(iii) Ethanol is liquid but ethyl chloride is gas at room temperature 🌡️
Ethanol forms strong intermolecular hydrogen bonds, while ethyl chloride cannot due to lack of polarized hydrogen (having only weak dipole–dipole forces). Therefore, ethanol is liquid and ethyl chloride is gas at room temperature.
Q5. Identify alcohol with two laboratory tests.
Answer
1) Identification of Alcohol by Sodium Metal Test ⚠️
Alcohol reacts with sodium metal to form sodium alkoxide with the liberation of hydrogen gas. The formation of brisk effervescence confirms the presence of an alcoholic (–OH) group.
Reaction: 2ROH + 2Na → 2RONa + H₂↑ (R = alkyl group)
2) Identification of Alcohol by Ester Test 🍍
When an alcohol is heated with acetic acid in the presence of concentrated sulphuric acid, an ester is formed. The appearance of a pleasant fruity smell indicates the presence of an alcoholic group.
Reaction: C₂H₅OH + CH₃COOH → CH₃COOC₂H₅ (fruity smell) + H₂O
Q6. What is Lucas reagent? Describe its use to distinguish between primary, secondary and tertiary alcohol.
Answer
✨Lucas reagent: a mixture of concentrated HCl and anhydrous ZnCl₂.
✨Purpose: Distinguishing primary, secondary, and tertiary alcohols based on the rate of formation of alkyl chloride (turbidity).
✨Basis: Faster turbidity = higher degree of alcohol
✨Order of reactivity of Alcohols: 3° > 2° > 1°⚡
✨Order of reactivity of Halogen acids: HI > HBr > HCl
(HCl reacts with less reactive alcohols only in presence of ZnCl₂)
✨Lucas Test Observations & Equations
⚡ (a) Tertiary alcohol 🚀
➡️Observation: Immediate turbidity / insoluble oily layer
➡️Condition: Room temperature
➡️Equation: R₃COH + HCl → R₃CCl + H₂O
⚡ (b) Secondary alcohol ⏱️
➡️Observation: Turbidity after 5–10 minutes
➡️Condition: Room temperature
➡️Equation: R₂CHOH + HCl → R₂CHCl + H₂O
⚡ (c) Primary alcohol 🔥
➡️Observation: No turbidity at room temperature
➡️Condition: No immediate reaction, occurs only on heating
➡️Equation: RCH₂OH + HCl → RCH₂Cl + H₂O (ZnCl₂, heat)
Q7. What is oxonium ion? How can ether form oxonium ion?
Answer
Oxonium Ion and its Formation 🧪
Oxonium ion is a positively charged ion in which the oxygen atom forms three bonds and carries a +1 formal charge (a trivalent oxygen cation). Its general formula is R₃O⁺ (R = H or alkyl group). The simplest oxonium ion is hydronium ion (H₃O⁺).
Formation of Oxonium Ion from Ether ⚡
Ethers act as weak bases due to the presence of lone pair of electrons on the oxygen atom. When an ether reacts with a strong acid, it accepts a proton to form an oxonium ion (oxonium salt).
Equation: R–Ӧ:–R′ + H⁺ — H₂SO₄ (conc.) → R– Ӧ⁺H–R′
Example Equation: R–Ӧ:–R′ — H⁺/HCl (conc.) → [R– Ӧ⁺H–R′]Cl⁻
Example Equation: R–Ӧ:–R′ — H⁺/H₂SO₄ (conc.) → [R– Ӧ⁺H–R′]HSO₄⁻
✏️ Smart Answers of Descriptive Questions ✏️
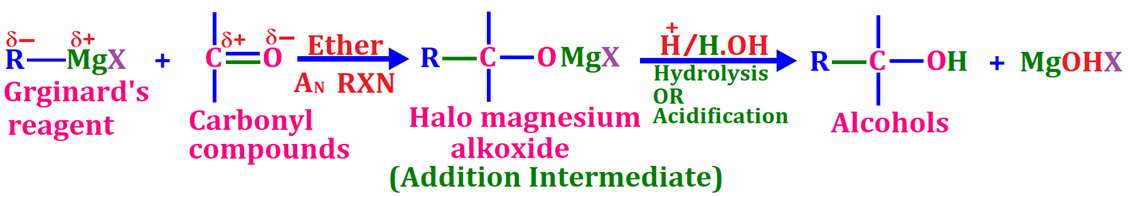
Q1. Starting from Grignard reagent how is primary, secondary and tertiary alcohol prepared?
Answer
Preparation of Three Types of Alcohols Using Grignard Reagent via its nucleophilic addition reaction with carbonyl compounds 🧪
Grignard reagents (RMgX) react with carbonyl compounds (formaldehyde, higher aldehydes like acetaldehyde and ketones like acetone) via nucleophilic addition to produce different types of next higher alcohols. The type of alcohol depends on the carbonyl compound used. The resulting alcohol has one more carbon atom than the Grignard reagent. (Grignard reagent acts as a nucleophile, attacking the electrophilic carbon of the carbonyl group forming magnesium alkoxide intermediate, which is converted to alcohol by acidic hydrolysis).
⚖️ General Reaction: Grignard + carbonyl → magnesium alkoxide (intermediate adduct) → hydrolysis → alcohol
Formaldehyde (H–CHO) → Primary Alcohols 🟢
Reaction: RMgX + H–CHO → [R–CH₂OMgX] → R–CH₂OH (after hydrolysis)
Higher aldehydes (e.g., acetaldehyde CH₃CHO) → Secondary Alcohols 🟡
Reaction: RMgX + R′–CHO → [R–CH(OMgX)–R′] → R–CH(OH)–R′ (after hydrolysis)
Ketones (e.g., acetone (CH₃)₂CO) → Tertiary Alcohols 🔴
Reaction: RMgX + R′₂C=O → [R–C(OMgX)–R′₂] → R–C(OH)–R′₂ (after hydrolysis)
📌 Summary
🟢 Primary alcohol: RMgX + H–CHO → R–CH₂OH
🟡 Secondary alcohol: RMgX + R′–CHO → R–CH(OH)–R′
🔴 Tertiary alcohol: RMgX + R′₂C=O → R–C(OH)–R′₂
Q2. Write the equations for the following possible conversions:
(i) Ethyl alcohol to diethyl ether
(ii) Phenol into benzoquinone
(iii) Ethyl bromide to ethanol
(iv) 2°-alcohol to carboxylic acid
(v) Ethanol into acetic acid
(vi) Ethyl alcohol into ethyne
(vii) Ethene into ethanol
Answer
✨Equations for Conversions
♻️ (i) Ethanol → Diethyl ether 🔥 (Condensation (intermolecular dehydration) of excess of Ethyl alcohol into diethyl ether in acidic medium at 140°C)
⚖️Equation: 2CH₃CH₂OH — (conc. H₂SO₄, 140°C) → CH₃CH₂–O–CH₂CH₃ + H₂O
♻️ (ii) Phenol → Benzoquinone ⚡ (Oxidation of Phenol into yellow coloured dicarbonyl compound benzoquinone)
⚖️Equation: C₆H₅OH + [O] — K₂Cr₂O₇/H⁺ → C₆H₄O₂ (oxidation)
♻️ (iii) Ethyl bromide → Ethanol 💧 (Sɴ of Br group of ethyl bromide by –OH group of aqueous KOH giving ethyl alcohol)
⚖️Equation: CH₃CH₂ᵟ⁺Brᵟ⁻ + K⁺OH⁻ (aq) — Sɴ₂ → CH₃CH₂OH + KBr
♻️ (iv) 2° Alcohol → Ketone → Carboxylic acid 🔄 (Oxidation of 2°-alcohol to lower previous carboxylic acid)
⚖️Equation: R₂CHOH + [O] — K₂Cr₂O₇/H⁺ → R₂C=O + 4[O] — K₂Cr₂O₇/H⁺ → RCOOH (strong oxid.)
♻️ (v) Ethanol → Acetic acid 🍎 (Oxidation of Ethanol with Acidified Dichromate first to aldehyde, then to acid)
⚖️Equations: CH₃CH₂OH + [O] — (K₂Cr₂O₇/H₂SO₄)/–H₂O → CH₃CHO + [O] — (K₂Cr₂O₇/H₂SO₄)/–H₂O → CH₃COOH
♻️ (vi) Ethyl alcohol → Ethyne 🔥 (Dehydration, Halogenation, Double Dehydrohalogenation)
CH₃CH₂OH — H₂SO₄/170°C/–H₂O → CH₂=CH₂ (Acid-catalyzed dehydration)
CH₂=CH₂ + Br₂ — CCl₄ → BrCH₂–CH₂Br (Halogenation)
BrCH₂–CH₂Br + 2KOH — Alcohol/Heat → CH≡CH + 2KBr + 2H₂O (Double Dehydrohalogenation)
♻️ (vii) Ethene → Ethanol 💧 (Acid-catalyzed Hydration of ethene into ethanol with Conc. via E₁ mechanism)
⚖️Equation: CH₂=CH₂ + H₂O — (H₂SO₄, 100°C) → CH₃CH₂OH
Q3. Differentiate between alcohol and phenol on the basis of:
(i) Solubility in water
(ii) Boiling point
(iii) Acidic character
Answer
✨ (i) Water solubility 💧
➡️Alcohol: Readily soluble due to hydrogen bonding with water
➡️Phenol: Less soluble; aromatic ring reduces water solubility
✨ (ii) Boiling Point 🔥
➡️Alcohol: Generally lower than phenols
➡️Phenol: Higher boiling point due to stronger hydrogen bonding
✨ (iii) Acidic Character / Acidity ⚡
➡️Alcohol: Weak acid (higher pKₐ)
➡️Phenol: Stronger acid (lower pKₐ)
Q5. Enlist the commercial applications of alcohol, phenol and ether.
Answer
Commercial Applications of Alcohols, Phenols, and Ethers ✨
Uses of Alcohols 🍾
Uses of Methanol (CH₃OH) 🔹
➡️ Perfumes, dyes, drugs
➡️ Antifreeze solution
➡️ Solvent for varnishes, paints, polishes
➡️ Production of formaldehyde / formalin
➡️ Mixed with ethanol → Methylated Spirit (denatured alcohol)
Uses of Ethanol (C₂H₅OH) 🔹
➡️ Raw material for organic compounds: acetone, acetic acid, esters, chloroform, ether, ethanol, resins, varnishes
➡️ Base for perfumes (Deodorized ethanol)
➡️ Low-temperature thermometric liquid
➡️ Petrol additive (octane improver)
➡️ Component of alcoholic beverages: spirits, wine, beer
Uses of Rubbing Alcohol (Isopropyl alcohol in water) 🔹
➡️ Used as antiseptic
Uses of Phenol (C₆H₅OH) ⚡
➡️ Antiseptic and disinfectant
➡️ Manufacturing of soaps, plastics, ointments, lozenges
➡️ Preparation of picric acid and phenolphthalein
➡️ Ink preservative
Uses of Ethers (R–O–R) 💧
➡️ Solvent in manufacturing waxes, gums, resins, oils
➡️ Solvent for Wurtz reaction & Grignard reagent preparation in the form of Diethyl ether
Q6. How do you prepare alcohol from carbonyl compounds, alkenes & esters?
Answer
Preparation of Alcohols from Carbonyl Compounds, Alkenes & Esters ✨
Preparation of Alcohols By the reduction of Carbonyl Compounds i.e. Aldehydes and ketones (Hydrogenation across >C=O)🔥
General Rule: Aldehydes → Primary alcohols
Ways of Reduction:
➡️ Catalytic reduction by H₂ gas using catalyst like Ni, Pt or Pd
➡️ Chemical reduction by nascent hydrogen using reducing agents like lithium aluminum hydride (LiAlH₄) or sodium borohydride (NaBH₄)
R–CHO + H₂ → R–CH₂OH (Ni/Pt/Pd, high temp)
or
R–CHO + [H] → R–CH₂OH (LiAlH₄ / NaBH₄)
Ketones → Secondary alcohols
R₂C=O + H₂ → R₂CHOH (Ni/Pt/Pd, high temp)
or
R₂C=O + [H] → R₂CHOH (LiAlH₄ / NaBH₄)
Preparation of Alcohols By Acid-catalyzed hydration of alkene 💧
Alkene + H₂O → Alcohol (conc. H₂SO₄, heat)
Example: CH₂=CH₂ + H₂O → CH₃CH₂OH
From Esters Using Grignard Reagent ⚡
Ester + RMgX → Ketone intermediate → Reacts with another RMgX → Tertiary alcohol
Equation (general):
RCOOR′ + RMgX → R₂C(OMgX)R′ → R₂C(OH)R′ (after hydrolysis)
From Esters Using LiAlH₄ 🔹
Ester + LiAlH₄ → Primary alcohols (two molecules of alcohol may form)
Equation:
RCOOR′ + 4[H] → RCH₂OH + R′OH
🧠 Key Points for Exams
Aldehydes → 1° alcohol
Ketones → 2° alcohol
Alkenes → Hydration → Alcohol
Ester + Grignard → 3° alcohol
Ester + LiAlH₄ → 1° alcohol
💥 غزل ۔۔۔۔ جونؔ ایلیا 💥
☀️ دھوپ آنگن میں پھیل جاتی ہے
🌆 شہر کوچوں میں خاک اڑاتی ہے
🕰️ میز پر گرد جمتی جاتی ہے
🌙 اب کسے رات بھر جگاتی ہے
💔 بے دلی بھی تو لب ہلاتی ہے
🌸 زندگی خواب کیوں دکھاتی ہے
💭 خواہشِ غیر کیوں ستاتی ہے
😮 ہمنشیں! سانس پھول جاتی ہے
👀 غور کرنے پہ یاد آتی ہے
💔 روز ایک چیز ٹوٹ جاتی ہے
▶ Watch Video on YouTube
Click the preview below to open in new window

▶ Click image to watch on YouTube