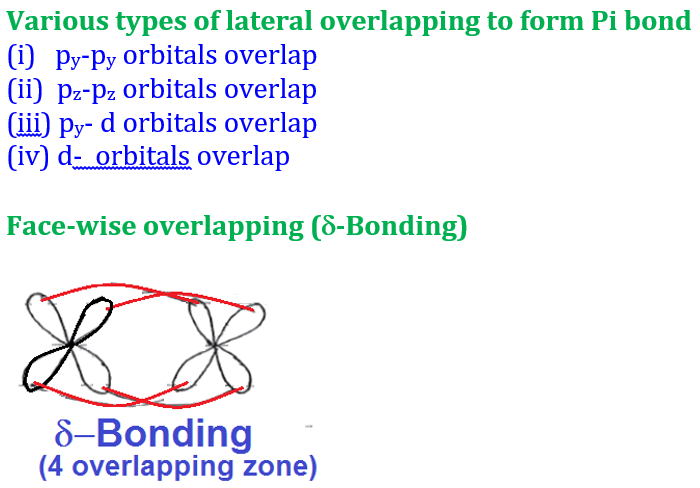
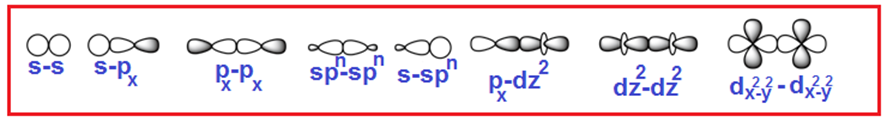
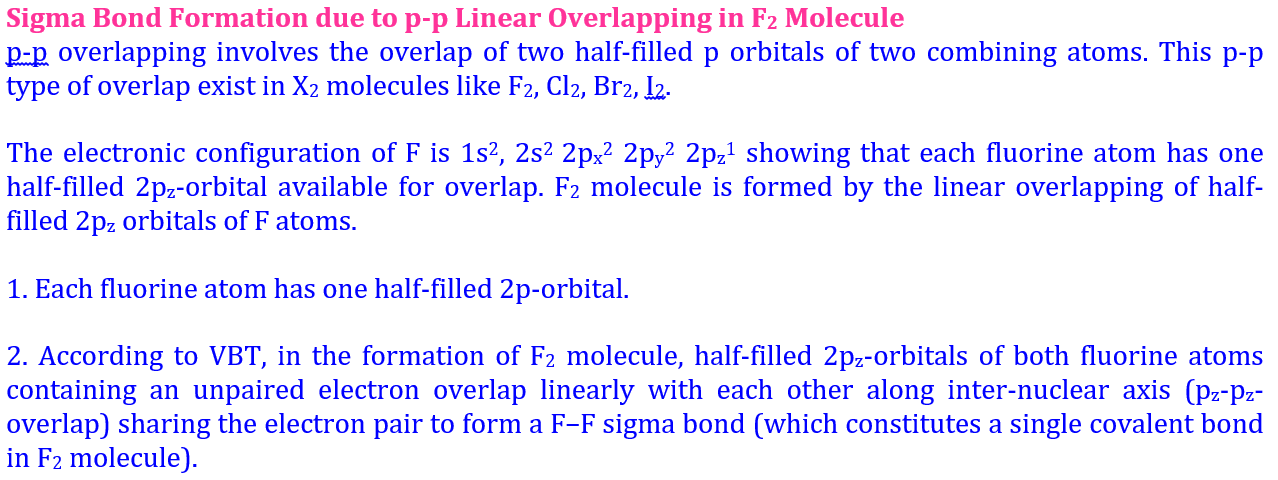
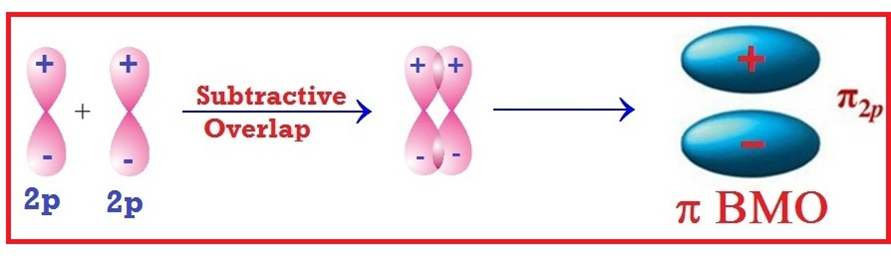
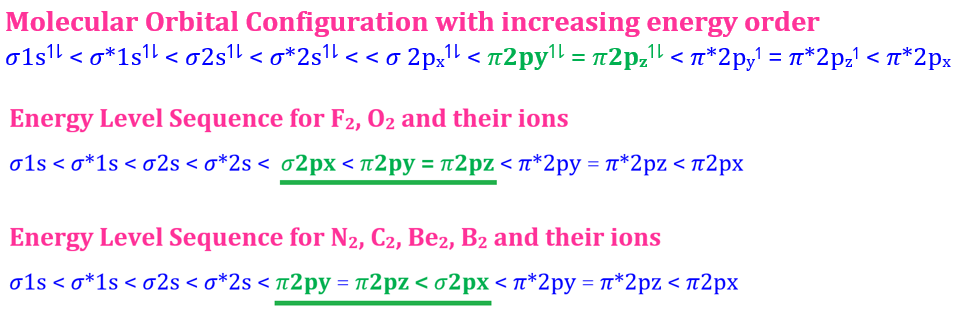
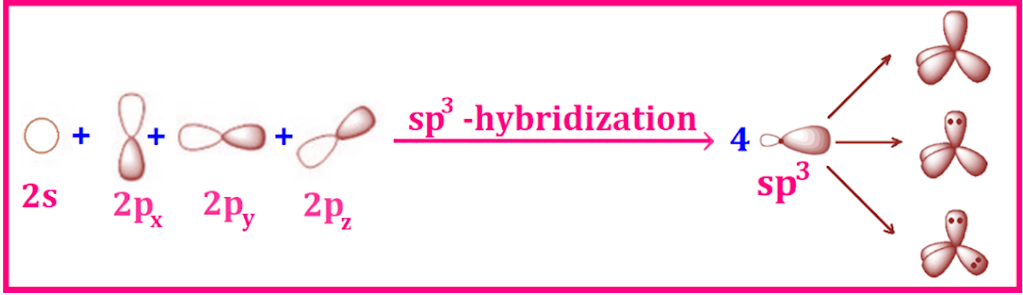
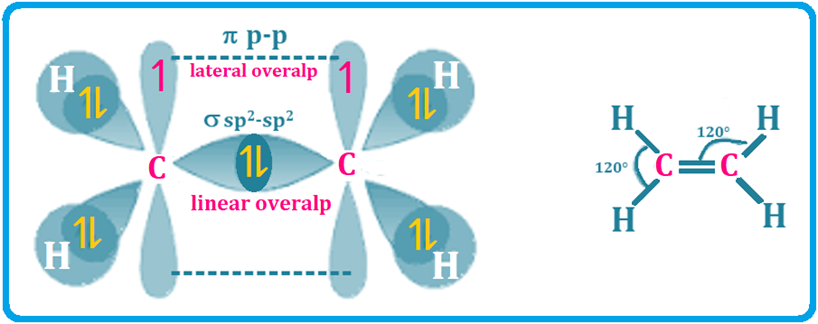
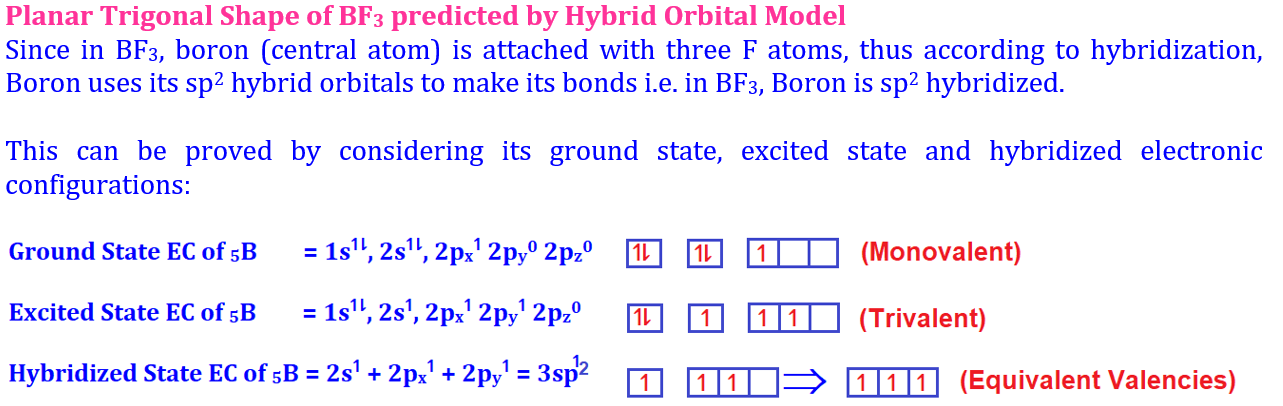
Welcome to Learn Chemistry by Inam Jazbi!
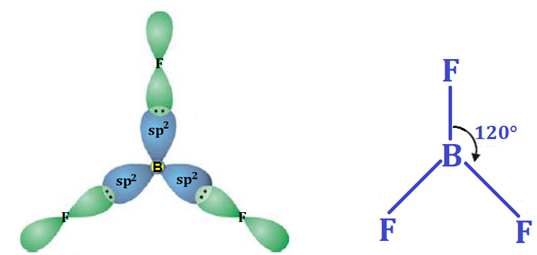
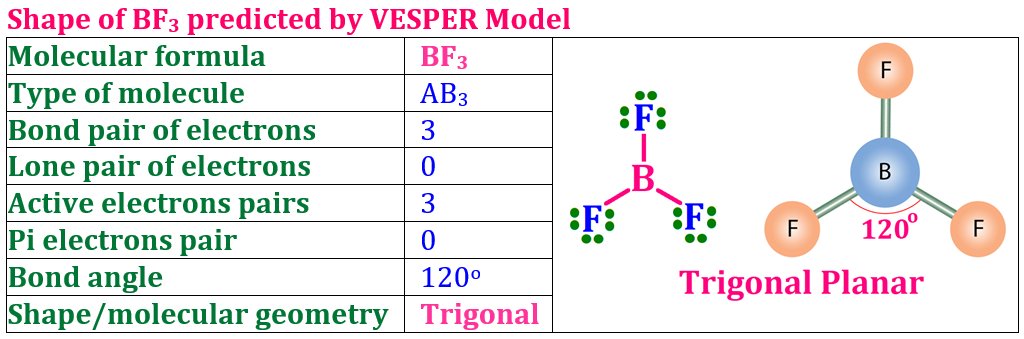
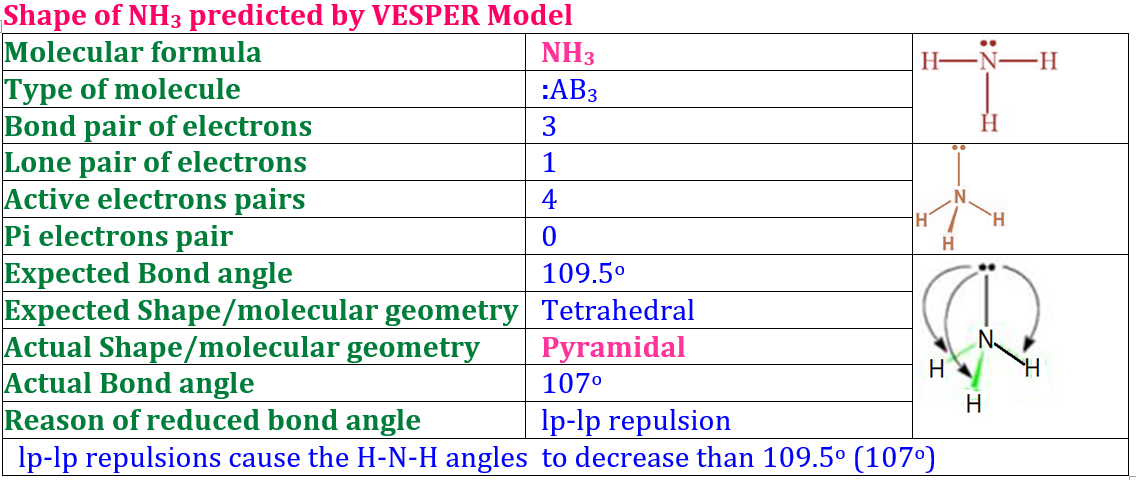
In this post, we’ll explore the Modern or Orbital Concept of Covalent Bond, one of the most important topics in Class XI Chemistry. This concept explains how atoms share electrons by the overlapping of atomic orbitals to form a stable molecule. You’ll learn about types of orbital overlap — s–s, s–p, and p–p overlapping, the difference between sigma (σ) and pi (π) bonds, and how the orbital theory gives a clearer picture than the classical concept. These notes are simplified and perfect for 2025 exam preparation.
Learn the Modern or Orbital Concept of Covalent Bond with easy explanations, diagrams, and examples. Perfect Class XI Chemistry notes for 2025 syllabus.
Modern or Orbital Concept of Covalent Bond
-
Orbital Overlap in Covalent Bonding
-
Sigma and Pi Bonds Explanation
-
Chemistry Notes for Class XI 2025
-
Covalent Bond Theory Class 11
#CovalentBond #OrbitalConcept #ChemistryNotes #XIClassChemistry #LearnChemistry #InamJazbi #ChemicalBonding #Chemistry2025