Get ready for your Class 9 Chemistry exam with Model Test #9 for Chapter 4: Chemical Bonding. Includes the most important conceptual, short, and MCQ questions based on the latest 2026 syllabus. Perfect for Sindh, Punjab, and Federal Board students.
IX Chemistry
⚡🧪 Chemistry Model Test #9 | Chapter 4 – Chemical Bonding (Important 2026 Questions & Answers) 🔗💥
🔥🌿Short Answer QuestionsQ1. Draw dot and cross diagrams to show how different types of chemical bonds are formed when fluorine reacts with (a) Hydrogen (b) potassium
Q2. What is meant by octet and duplet rule?
Q3. Can you draw an ion which is formed by the atom losing three electrons?
Q4. How oxygen forms an anion?
Q5. What is the difference between lone pair and bond pair?
Q6. Explain why table salt has a very high melting point.
Q7. How is electronegative value determined the formation of chemical bond?
Q8. Why is to easy for magnesium atom to lose two electrons?
Q9. Atoms of metallic elements can form ionic bond, but they are not very good to form covalent bonds. Why?
Q10. How does an ion differ from an atom?
Q11. Describe dipole-dipole forces.
Q12. Write uses of adhesive material.
Q13. Why Intermolecular forces are weaker than intra molecular forces?
Q14. Write characteristics of metallic bond.
Q15. Covalent bonds are strong and hard to break but why most of the covalent compounds have low melting and boiling points.
Q16. Write down the characteristics of ionic compounds.
Q17. Why ionic compounds are solid?
Q18. How is hydrogen bonding affecting the physical properties of compounds?
Q19. Explain element attain stability?
Q20. Define metallic bond. How are metallic bonds formed?
🔥🌿Long Answer Questions
Q1. Define ionic bond. Discuss the formation of sodium chloride (NaCl).
Q2. What is meant by covalent bond? Describe the formation of a covalent bond between two nonmetallic atoms. Explain single, double and triple covalent bond with examples.
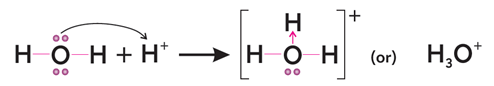
Q4. What is coordinate covalent bond? Explain with two examples.
Q5. What do you understand about ionic character of covalent bond?
Q6. Differentiate the properties of polar and non-polar compounds.
Q7. Explain the importance of glues and epoxy resins in our society.
Q8. Give difference between following:
a) Ionic compounds and covalent compounds (b) Polar and non-polar bond
c) Covalent and co-ordinate bond. (d) Ionic and covalent bond.
Q9. What is hydrogen bonding? What type of forces, either intramolecular or intermolecular forces are present in hydrogen bond? Explain the origin of hydrogen bonding?
💡🧪 Solutions of Model Test Questions | Ace Your Chemistry Exams with Step-by-Step Answers! ✨📘🧬
Important
Question
🧪⚡ Chemical Bonding MCQs | Master Ionic, Covalent & Hydrogen Bonds with Fun! 🌈📘✨
1️⃣ An example of ionic compound is:🟨 A H₂
🟩 B CH₄
🟦 C NaCl
🟪 D N₂
2️⃣ Interaction between highly electron-deficient hydrogen and highly electronegative atom is called:
🟨 A Ionic bond
🟩 B Metallic bond
🟦 C Hydrogen bond
🟪 D Covalent bond
3️⃣ Two fluorine atoms share one electron each in their outermost shell to achieve electronic configuration of:
🟨 A Xe
🟩 B Ar
🟦 C Kr
🟪 D Ne
4️⃣ Number of electrons lost by atoms of group IIIA equals:
🟨 A 1
🟩 B 2
🟦 C 3
🟪 D 4
5️⃣ Atom which loses two electrons from its outer shell to form ion is called:
🟨 A Oxygen
🟩 B Potassium
🟦 C Magnesium
🟪 D Carbon
6️⃣ In NaCl crystal lattice each Na⁺ ion is surrounded by:
🟨 A 6 Cl⁻ ions
🟩 B 6 Na⁺ ions
🟦 C 8 Cl⁻ ions
🟪 D 12 Cl⁻ ions
7️⃣ At room temperature most of ionic compounds are:
🟨 A Amorphous solids
🟩 B Crystalline solids
🟦 C Liquids
🟪 D Gases
8️⃣ Tendency of atoms to acquire eight electrons in their valence shell is:
🟨 A Octet rule
🟩 B Duplet rule
🟦 C Triplet rule
🟪 D None of above
9️⃣ When one atom forms cation by losing electron and other forms anion by accepting that electron, the bond formed is:
🟨 A Coordinate covalent bond
🟩 B Covalent bond
🟦 C Ionic bond
🟪 D Hydrogen bond
🔟 Noble gases are stable because they contain ………. electrons in valence shell:
🟨 A 4
🟩 B 6
🟦 C 8
🟪 D 10
1️⃣1️⃣ Bond which involves 3 shared electron pairs is a:
🟨 A Double covalent bond
🟩 B Single covalent bond
🟦 C Triple covalent bond
🟪 D None of above
1️⃣2️⃣ A non-metal atom forms an anion by:
🟨 A Losing electrons
🟩 B Gaining electrons
🟦 C Losing protons
🟪 D Gaining protons
1️⃣3️⃣ When two identical atoms share electron pairs and exert force on each other, the bond formed is:
🟨 A Coordinate covalent bond
🟩 B Non-polar covalent bond
🟦 C Double covalent bond
🟪 D Polar bond
1️⃣4️⃣ Synthetic resins are used on places where:
🟨 A Electric resistance is required
🟩 B Water resistance is required
🟦 C Adhesion is required
🟪 D Friction is required
1️⃣5️⃣ Oxygen belongs to group VIA, so number of electrons in its valence shell are:
🟨 A 3
🟩 B 4
🟦 C 5
🟪 D 6
1️⃣6️⃣ Electron pairs which are not shared by atoms are called:
🟨 A Bond pair
🟩 B Lone pairs
🟦 C Shared pairs
🟪 D Electron pairs
1️⃣7️⃣ Strength of intermolecular forces from ionic or covalent bond is:
🟨 A Weaker
🟩 B Stronger
🟦 C Equal
🟪 D None of above
1️⃣8️⃣ Ionic crystals have:
🟨 A High melting points
🟩 B Moderate melting points
🟦 C Low melting points
🟪 D None of above
1️⃣9️⃣ Bond formed by mutual sharing of electrons is:
🟨 A Coordinate covalent bond
🟩 B Covalent bond
🟦 C Metallic bond
🟪 D Ionic bond
2️⃣0️⃣ Which of the following diagram shows atoms bonded with same electronegativity?
🟨 A
🟩 B
🟦 C
🟪 D
2️⃣1️⃣ The forces which hold atoms together in a molecule are called:
🟨 A Coordinate covalent bond
🟩 B Electrovalent bond
🟦 C Chemical bond
🟪 D Covalent bond
2️⃣2️⃣ ………… covalent molecule is electrically neutral as well as symmetrical:
🟨 A Dative
🟩 B Covalent
🟦 C Non-polar
🟪 D Polar bond
2️⃣3️⃣ The power of an atom to attract the shared pair of electrons towards itself is called:
🟨 A Electronegativity
🟩 B Ionization energy
🟦 C Electron affinity
🟪 D Electropositivity
2️⃣4️⃣ ………. compounds are usually made up of discrete units with weak intermolecular forces:
🟨 A Electrovalent
🟩 B Covalent
🟦 C Co-ordinate bond
🟪 D Polar
2️⃣5️⃣ NaCl is …………. compound:
🟨 A Electrovalent
🟩 B Covalent
🟦 C Co-ordinate covalent
🟪 D Polar
2️⃣6️⃣ The bond in MgO is:
🟨 A Electrovalent bond
🟩 B Co-ordinate covalent bond
🟦 C Chemical bond
🟪 D Covalent bond
2️⃣7️⃣ If electronegativity difference of bonded atoms is more than 1.7, the bond is …………:
🟨 A Electrovalent bond
🟩 B Co-ordinate covalent bond
🟦 C Polar bond
🟪 D Covalent bond
2️⃣8️⃣ It is the electrostatic attraction between positive ions and the electrons of the atoms:
🟨 A Electrovalent bond
🟩 B Covalent bond
🟦 C Chemical bond
🟪 D Metallic bond
🟦 C London forces
🟪 D IMF
3️⃣0️⃣ CO₂ is ………… molecule:
🟨 A Co-ordinate covalent
🟩 B Covalent
🟦 C Polar
🟪 D Electrovalent
3️⃣1️⃣ The atom which accepts a lone pair of electron is called …………..:
🟨 A Acceptor
🟩 B Donor
🟦 C Receiver
🟪 D None of these
3️⃣2️⃣ The force which holds atoms together in a molecule or crystal is called:
🟨 A Coordinate covalent bond
🟩 B Covalent bond
🟦 C Chemical bond
🟪 D Ionic bond
3️⃣3️⃣ This bond is formed by the transfer of one or more electrons from one atom to another atom:
🟨 A Ionic bond
🟩 B Co-ordinate covalent
🟦 C Chemical bond
🟪 D Covalent bond
3️⃣4️⃣ The bond formed by mutual sharing of electrons between the atoms is called:
🟨 A Co-ordinate covalent bond
🟩 B Electrovalent bond
🟦 C Covalent bond
🟪 D Chemical bond
3️⃣5️⃣ The bond formed by one-sided sharing of pair of electrons is called:
🟨 A Co-ordinate covalent bond
🟩 B Covalent bond
🟦 C Chemical bond
🟪 D Ionic bond
3️⃣6️⃣ The shared pair of electrons which links the atoms in a molecule is known as:
🟨 A Co-ordinate covalent bond
🟩 B Covalent bond
🟦 C Electrovalent bond
🟪 D Chemical bond
3️⃣7️⃣ Double covalent bond is denoted by:
🟨 A Single short line
🟩 B Two short lines
🟦 C Three short lines
🟪 D None of these
3️⃣8️⃣ The atom which supplies the pair of electrons for bond formation is known as:
🟨 A Acceptor
🟩 B Donor
🟦 C Receiver
🟪 D None of these
3️⃣9️⃣ Co-ordinate covalent bond is always formed between the two:
🟨 A Like & unlike atoms
🟩 B Unlike atoms
🟦 C Similar atoms
🟪 D Like atoms
4️⃣0️⃣ The shared pair of electrons in a co-ordinate covalent bond is denoted by:
🟨 A A single line
🟩 B Double line
🟦 C An equal sign
🟪 D An arrow
✅ Answers – Chemical Bonding MCQs
1️⃣ 🟪 D – NaCl
2️⃣ 🟩 C – Hydrogen bond
3️⃣ 🟦 D – Ne
4️⃣ 🟨 C – 3
5️⃣ 🟨 C – Magnesium
6️⃣ 🟨 A – 6 Cl⁻ ions
7️⃣ 🟨 B – Crystalline solids
8️⃣ 🟩 A – Octet rule
9️⃣ 🟦 C – Ionic bond
🔟 🟦 C – 8 electrons
1️⃣1️⃣ 🟦 C Triple covalent bond
1️⃣2️⃣ 🟩 B Gain of electrons
1️⃣3️⃣ 🟩 B Non-polar covalent bond
1️⃣4️⃣ 🟨 A Electric resistance
1️⃣5️⃣ 🟪 D 6
1️⃣6️⃣ 🟩 B Lone pairs
1️⃣7️⃣ 🟩 B Stronger
1️⃣8️⃣ 🟨 A High melting points
1️⃣9️⃣ 🟩 B Covalent bond
2️⃣0️⃣ 🟨 A
2️⃣1️⃣ 🟦 C Chemical bond
2️⃣2️⃣ 🟦 C Non-polar
2️⃣3️⃣ 🟨 A Electronegativity
2️⃣4️⃣ 🟩 B Covalent
2️⃣5️⃣ 🟨 A Electrovalent
2️⃣6️⃣ 🟨 A Electrovalent bond
2️⃣7️⃣ 🟨 A Electrovalent bond
2️⃣8️⃣ 🟪 D Metallic bond
2️⃣9️⃣ 🟩 B Hydrogen bond
3️⃣0️⃣ 🟩 B Covalent
3️⃣1️⃣ 🟨 A Acceptor
3️⃣2️⃣ 🟦 C Chemical bond
3️⃣3️⃣ 🟨 A Ionic bond
3️⃣4️⃣ 🟦 C Covalent bond
3️⃣5️⃣ 🟨 A Co-ordinate covalent bond
3️⃣6️⃣ 🟩 B Covalent bond
3️⃣7️⃣ 🟩 B Two short lines
3️⃣8️⃣ 🟩 B Donor
3️⃣9️⃣ 🟩 B Unlike atoms
4️⃣0️⃣ 🟪 D An arrow
🧠 Related Posts:
https://learnchemistrybyinamjazbi.blogspot.com/2025/05/ix-chemistry-notes-2025-new-concise.html
https://learnchemistrybyinamjazbi.blogspot.com/2025/02/ix-chemistry-important-mcqs-of-text.html
https://learnchemistrybyinamjazbi.blogspot.com/2025/02/x-chemistry-mcqs-for-board-paper-2025.html
https://learnchemistrybyinamjazbi.blogspot.com/2024/08/ixxii-valency.html
https://learnchemistrybyinamjazbi.blogspot.com/2024/07/ix-conceptual-chemistry-book-by-dr-inam.html
https://learnchemistrybyinamjazbi.blogspot.com/2024/07/ix-model-test-questions-chemistry-test.html
https://learnchemistrybyinamjazbi.blogspot.com/2024/06/ix-model-test-questions-class-9.html
https://learnchemistrybyinamjazbi.blogspot.com/2024/05/fundamentals-of-chemistry.html
https://learnchemistrybyinamjazbi.blogspot.com/2024/03/ix-chemistry-model-test-questions-of.html
https://learnchemistrybyinamjazbi.blogspot.com/2025/11/ix-chemistry-guess-paper-2026-100-board.html
https://learnchemistrybyinamjazbi.blogspot.com/2025/02/ix-chemistry-important-mcqs-of-text.html



















































Wania Qadeer
ReplyDeleteSir,
Your blog was really easy to understand. It helped me a lot.