Learn what stereoisomerism is, its main types (geometrical and optical isomerism), and see clear examples for each. Perfect for Class XII Chemistry students preparing for board exams.
What is Stereoisomerism?
🔹 Difference between Structural and Stereoisomerism
| Basis | Structural Isomerism | Stereoisomerism |
|---|---|---|
| Definition | Atoms are connected in different ways. | Atoms are connected in the same way but arranged differently in space. |
| Example | Butane & Isobutane | cis-But-2-ene & trans-But-2-ene |
🧪 Types of Stereoisomerism
There are two main types of stereoisomerism:
1. Geometrical (cis–trans) isomerism
2. Optical isomerism
🔸 1. Geometrical Isomerism (cis–trans Isomerism)
-
Found in alkenes and compounds with restricted rotation (like double bonds).
-
Caused by different spatial arrangements of groups around a double bond.
Example:
👉 cis-But-2-ene and trans-But-2-ene
| Isomer | Structure | Property |
|---|---|---|
| cis-isomer | Similar groups on the same side of the double bond | Higher boiling point |
| trans-isomer | Similar groups on opposite sides of the double bond | More stable |
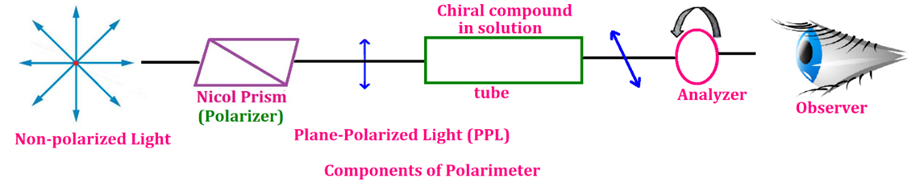
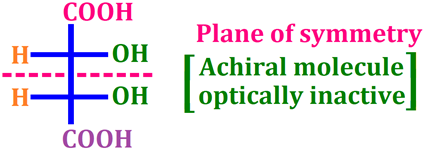
🔸 2. Optical Isomerism
-
Found in compounds containing a chiral carbon atom (a carbon attached to four different groups).
-
These isomers are non-superimposable mirror images of each other.
-
They rotate plane-polarized light in opposite directions.
Example:
👉 Lactic acid or Tartaric acid
| Isomer | Rotation of Light | Description |
|---|---|---|
| D-form | Rotates light to the right (+) | Dextrorotatory |
| L-form | Rotates light to the left (–) | Levorotatory |
🧭 Summary Chart
| Type | Example | Key Feature |
|---|---|---|
| Geometrical | cis–trans isomers | Restricted rotation |
| Optical | D–L isomers | Chirality (chiral center) |
📘 In Short:
Stereoisomerism = Same formula, different spatial arrangement.
Stereoisomerism Without Confusion | Best Complete Class 12
Chemistry Notes with Types, Tricks, Diagrams
& Examples for Board & MDCAT
💥 غزل جونؔ ایلیا (کیوں سے) 💥
🖋️ دیباچئہ وجود پہ لا لکھ رہا ہوں میں
✨ جو بھی نہیں ہے اس کو خدا لکھ رہا ہوں میں
🪞 ہے یوں کہ خود کوخود سے جدا لکھ رہا ہوں میں
🖊️ اپنے ہر ایک قول میں یا لکھ رہا ہوں میں
👕 خلوت میں رمزِ بندِ قبا لکھ رہا ہوں میں
⚔️ ہاں داستانِ کرب و بلا لکھ رہا ہوں میں
🍃 خاشاک کو متاعِ صبا لکھ رہا ہوں میں
🌌 عیار ہوں خدا کو خدا لکھ رہا ہوں میں
🌠 ہاں صفحئہ فضا پہ خلا لکھ رہا ہوں میں
🔮 یکتا ہے تُو سو تجھ کو دوتا لکھ رہا ہوں میں
🌕 مہتاب ! آج تجھ کو سُہا لکھ رہا ہوں میں
📖 رُودادِ جرگئہ امرا لکھ رہا ہوں میں
💨 اک عمر سے ہوا کو ہوا لکھ رہا ہوں میں
👸 بلقیس تجھ کو ننگِ سبا لکھ رہا ہوں میں
🔔 اور اس تمام تر میں صدا لکھ رہا ہوں میں
🖊️ کیا لکھ رہا ہوں، جانیے کیا لکھ رہا ہوں میں
🕌 دیر و حرم کو عقدہ کشا لکھ رہا ہوں میں
🤔 یہ بات مجھ کو بھی نہ بتا لکھ رہا ہوں میں
⚰️ تیری فنا اور اپنی بقا لکھ رہا ہوں میں
📜 پس ردِ گفتئہ جہلا لکھ رہا ہوں میں
🌌 اس لمحے سے فنا کی بقا لکھ رہا ہوں میں
🖋️ رُودادِ لكنتِ فصحا لکھ رہا ہوں میں
⚔️ تم سب کے نام اے جہلا لکھ رہا ہوں میں
🌀 اب حیلہ سازئ سفرا لکھ رہا ہوں میں
📖 اسمائے رستئہ نقبا لکھ رہا ہوں میں
💫 اک حال بے صباح و مسا لکھ رہا ہوں میں