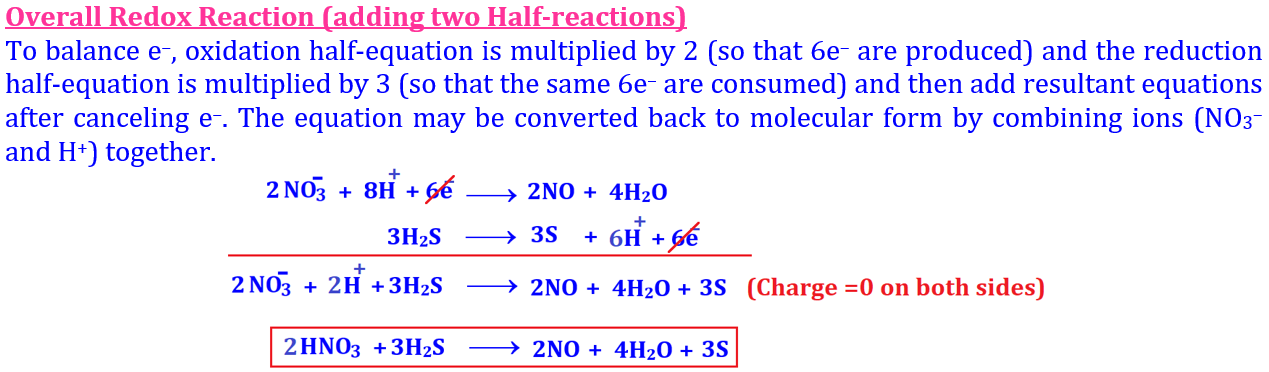
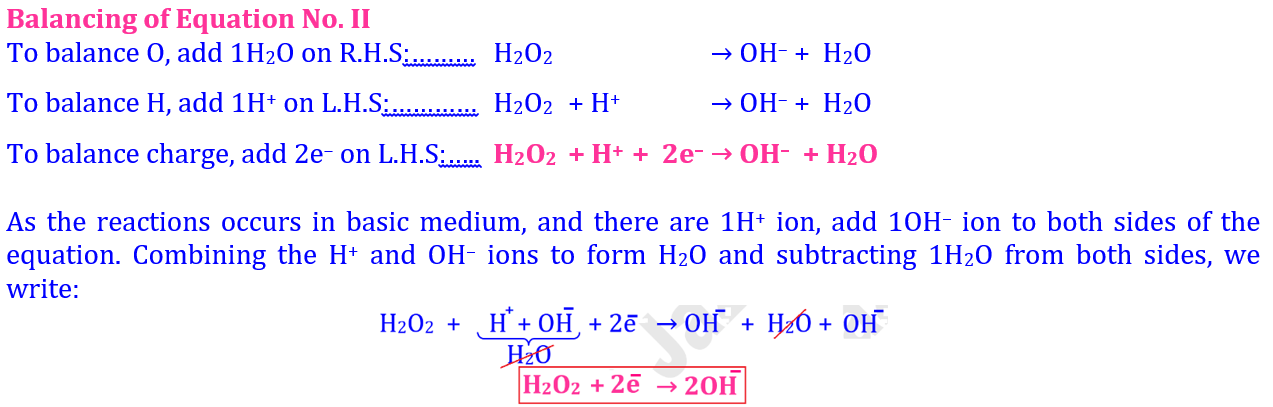
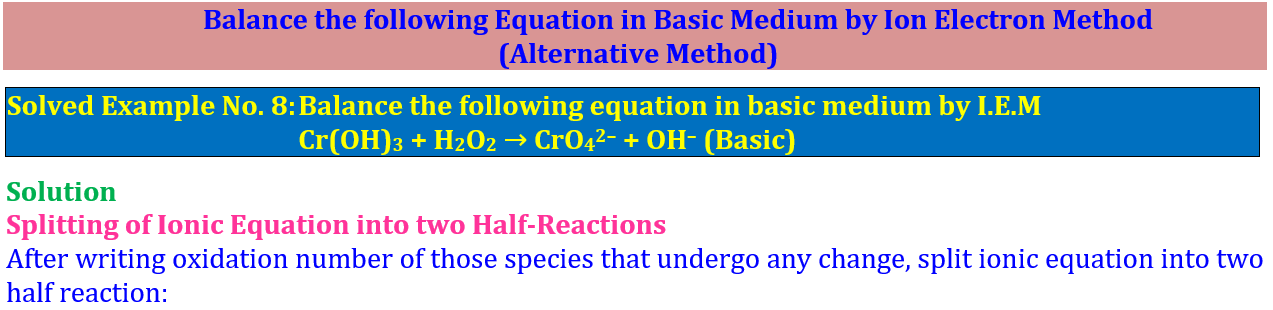
According to Loss or Gain of Electrons 💥 🔥
🔥💥Difference between Oxidation and Reduction
🔥💥Difference between Oxidation and Reduction (Short Version)
1. Oxidation
as addition of oxygen
Oxidation is defined as a reaction of addition of oxygen to a substance either other elements or compounds (to produce oxide) e.g. the rusting of iron, burning of magnesium, carbon etc. in air are typical examples of oxidation.
2. Oxidation as a removal of Hydrogen
Oxidation is the process of removal of hydrogen from a
compound. In organic chemistry, removal of hydrogen from a compound is termed
as dehydrogenation.
3. Oxidation
as addition of electronegative element
Oxidation is a chemical reaction in which an electronegative
element is added into any chemical species (atom, molecule or ion).
4. Oxidation
as Removal of electropositive element
Oxidation is a chemical reaction in which an electropositive element is removed from any chemical species (atom, molecule or ion).
5. Oxidation
as loss or removal of electrons (Electronic definition)
The most concise definition and broader view of oxidation reaction is in terms of the electron transfers.
According
to modern electronic concept, the
process or a reaction in which a substance (i.e. atom, molecule or ion) loses one or more electrons (which is
manifested by an increase in its oxidation number) is called Oxidation. Oxidation involves in producing or increasing the positive
charge on the species or decreasing its negative charge
e.g.
The substance that donates electrons is oxidized but it acts as a
reducing agent.
6. Oxidation as an increase in oxidation number
In terms
of oxidation number concept, the process in which the oxidation state of an
element is increased is called Oxidation.
e.g.
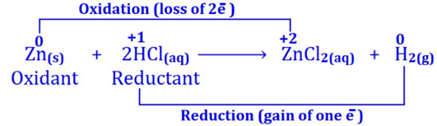
(i) In the following redox reaction between zinc powder and
dilute hydrochloric acid, zinc has been oxidized
to zinc chloride because its oxidation number has
been increased from 0 to +2.
(ii) In the following redox reaction between carbon and oxygen gas, carbon has been
oxidized to carbon dioxide because its oxidation number has been increased from 0 to +4.
Reduction or Electronation
1. Reduction as
addition of hydrogen
Reduction is the process of addition of
hydrogen to a
substance. In organic chemistry, addition of hydrogen in a substance is termed
as hydrogenation.
2. Reduction as
removal of oxygen
Reduction is the process or a reaction (just opposite to oxidation) which involves the removal of oxygen (or electronegative atom) from substances (e.g. oxides).
The most of such reactions are simple displacement reactions. In such reactions, oxides are reduced to free elements (usually metal) while other substances (usually an element) are oxidized to their respective oxides.
3. Reduction as
addition of an electropositive element
Reduction is a chemical reaction in which an electropositive element is added to any chemical species (atom, molecule or ion).
4. Reduction as
removal of an electronegative element
Reduction is a chemical reaction in which an electronegative element is removed from chemical species (atom, molecule or ion).
5. Reduction as
gain or addition of electrons (Electronic definition)
The most concise definition and broader view of reduction reactions is in terms of the electron transfers.
According
to modern electronic concept, the
process or a reaction in which a substance (i.e. atom, molecule or ion) gains one or more electrons (which is
manifested by a decrease in its oxidation number) is called Reduction. Reduction involves in producing or increasing the negative
charge on the species or decreasing its positive charge
The substance that gains electrons is reduced but it acts as an oxidizing
agent.
e.g
6. Reduction as a decrease in oxidation number
In terms
of oxidation number concept, the
process in which the oxidation state of an element is decreased is
called Reduction
e.g.
In this reaction, reduction of Br₂ occurs due to decrease in its oxidation number from 0 to –1.
Oxidizing Agent or Oxidant OR Oxidizer
Complete definition
In any redox reaction, the specie that oxidizes the other substance and itself gets reduced is known as oxidizing agent. It is a substance which gains electrons during a reaction from other substance undergoing decrease in oxidation number thereby oxidizing it. Stronger oxidizing agents are found in the lower region of ECS. Thus strongest oxidizing agent is fluorine.
Oxidizing agent may be defined as a substance supplying oxygen
or electronegative element, removing
hydrogen or electropositive element and accepting
electrons thereby decreasing oxidation number. Oxidizing
agent
1. Gives
nascent oxygen
2. accepts hydrogen
3. gains one or more electrons
4. undergoes decrease in oxidation number
5. causes oxidation
6. is reduced
1. Oxidizing Agent as donor of oxygen
Oxidizing agent is a substance (element or compound) that releases or supplies oxygen or nascent (or atomic) oxygen either on
decomposition or on treatment with other substance.
e.g.
in following reaction, CuO being donor of oxygen is acting as oxidizing agent supplying oxygen to H₂ and thus itself reduces to Cu while H₂ being acceptor of oxygen is acting as reducing agent adding oxygen and thus itself oxidizes to H₂O.
Some other examples of oxidant are illustrated by their decomposition or their reactions with other reagents.
2. Oxidizing Agent as Acceptor of Hydrogen
Oxidizing agent is a substance that removes or accepts hydrogen from a substance.
3. Oxidizing Agent as Electron Acceptor or Electron
recipient
A substance (in a redox chemical reaction) that accepts or
gains or receives one or more electrons from other substance (called the
reductant or reducer) is known an oxidizing agent or oxidant. Stated differently, oxidizing agent
undergoes decrease in oxidation number. Thus it is a substance that oxidizes the other substance (by removing
electrons from it) while itself gets reduced (by accepting electrons from the
other substance) to a lower oxidation state.
e.g.
(i) Br₂ molecule accepts electron during reaction and thus it acts as an oxidant.
(ii) Cl₂ molecules decreases its oxidation number from 0 to −1, so it acts as an oxidizing agent.
(iii) zinc reacts with dilute sulphuric acid to form zinc sulphate and hydrogen gas. In this redox reaction, H₂SO₄ acts as an oxidizing agent accepting electrons (undergoing increase in oxidation number) form zinc and thus reduces to H₂ gas while zinc acts as reducing agent by donating electrons (undergoing decreases in oxidation number) and thus oxidizes to Zn2+ ions. [SO₄2– ions being spectator ions, do not appear in net equation].
4. Test for Oxidizing Agent by KI solution
The resulting colour change of colourless to brown by the addition of KI (a reductant) to an oxidizing agent is a test for oxidizing agent.
Aqueous potassium iodide (KI) is used to test for the presence of an oxidizing agent. KI is colourless. If a drop of KI is added to a solution containing an oxidizing agent, a brown solution will be formed. The solution turns brown because the iodide ions (I-) is oxidized to iodine (I₂), by the oxidizing agent. The iodide ion is colourless but aqueous iodine is brown.
Starch-iodide paper can also be used to test for the presence of oxidizing agents. Oxidizing agents change the colour of most starch-iodide paper from white to blue. This is because the iodine produced reacts with the starch to give a blue colour.
5) Examples of Oxidizing Agent
Following are the examples of Oxidant:
Reducing Agent or Reductant or Reducer
Complete
Definition
In any redox reaction, the specie that reduces the other substance and itself gets oxidized is known as Reducing Agent or Reductant. It is a substance which loses electrons and give to the other for reduction undergoing oxidation. Strongest reducing agent are located on upper region of ECS. Thus strongest reducing agent is Li.
A reducing agent may be defined as a substance supplying hydrogen or
electropositive element, removing
oxygen or electronegative element and donating electrons thereby increasing oxidation number. Reducing agent
1. accepts
nascent oxygen
2. loses
hydrogen
3. loses one
or more electrons
4. oxidation
number of atom increases
5. causes
reduction
6. is oxidized
1.
Reducing Agent as Acceptor of Oxygen
Reducing agent is a substance (element or component) that accepts oxygen or nascent
(or atomic) oxygen (released by
oxidizing agent).
e.g.
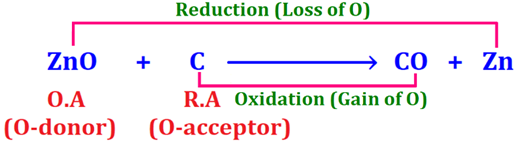
in following reaction, C being acceptor of oxygen is acting as reducing agent receiving oxygen from ZnO and thus itself oxidizes to CO while ZnO being donor of oxygen is acting as oxidizing agent supplying oxygen and thus itself reduces to Zn.
2. Reducing Agent as Donor of Hydrogen
Reducing agent is a substance (element or compound) that releases nascent (or atomic) hydrogen either on decomposition or on treatment with other substance.
3) Reducing Agent as
Electron Donor
A substance that donates or loses one or more electrons is called a reducing agent or reductant.
Thus it is a substance that reduces the other substance (by supplying electrons
to it) while itself gets oxidized (by losing electrons). Stated differently, reducing
agent undergoes increase in oxidation number during a
reaction.
e.g.
(i) Zn atom loses electrons during reaction & thus it is a reducing agent.
(ii) Na atom loses electrons during reaction & thus it is a reducing agent.
(iii) Magnesium reacts with dilute sulphuric acid to form magnesium sulphate and hydrogen gas. In this redox reaction, H₂SO₄ acts as an oxidizing agent accepting electrons (undergoing increase in oxidation number) from magnesium and thus reduces to H2 gas while magnesium acts as reducing agent by donating electrons (undergoing decreases in oxidation number) and thus oxidizes to Mg2+ ions. [SO₄²⁻ ions being spectator ions, do not appear in net equation].
4) Identification
Test for Reducing Agent by acidified potassium dichromate
The resulting colour change of orange to green by the addition of acidified potassium dichromate (an oxidant) to a reducing agent can be used as a test for reducing agent.
Acidified potassium dichromate (VI) can be used to test for the presence of a reducing agent. Acidified potassium dichromate (VI) is made by adding dilute sulphuric acid to aqueous potassium dichromate (VI). The colour of Acidified potassium dichromate (VI) solution changes from orange to green in the presence of a reducing agent. The half reaction is shown here:
In this reaction, the dichromate (VI) ion (Cr₂O₇²⁻) is reduced to the chromium (III) ion (Cr3+). Cr₂O₇²⁻ loses oxygen and the oxidation state of chromium decreases from +6 to +3.
5) Examples of Reducing Agent
Following are
the examples of Reductant:
➡️1. Non-reactive non-metals …✨e.g. C, H₂, S
➡️2. All metals …………………✨e.g. Li, K, Na, Al, Mg, Zn, Cu etc.
➡️3. Few acids (binary acids) …✨e.g. HCl, HBr, HI, H₂S, HCOOH, ascorbic acid, H₃PO₃, H₂C₂O₄ etc.
➡️ 4. Ionic Hydrides ……………✨ e.g. NaH, CaH₂ etc.
➡️ 5. Complex Hydrides ………..✨ e.g. LiAlH4, NaBH₄
➡️ 6. Miscellaneous ……………✨ e.g. SO₂,
CO, H₂O₂, NH₃, iodides (KI), ferrous salts
(FeSO₄, Mohr’s salt), stannous salts, cyanides salts, Na₂S₂O₃,
Na₂SO₃, hydrazine etc.
➡️ 7. Organic reducing agent ……✨ e.g. Formaldehyde, glucose etc.
➡️ 8. Metal alloys …✨ e.g. Sodium amalgam (Na-Hg), Zinc amalgam (Zn-Hg), Sodium-lead alloy (Na + Pb)
Difference between Oxidizing and Reducing Agent
Criteria of deciding oxidizing and reducing nature of compounds
A substance acts only as an oxidizing if the oxidation
number of one of its element (central atom) is in its highest oxidation state and as a reducing agent if the oxidation number of one of
its element is in its lowest oxidation state. However, if the oxidation number of one of the elements
of a substance is in its intermediate oxidation state, it can act both as an oxidizing as well as a reducing
agent.
1. The oxidation number of N in HNO₃ is
maximum i.e.+5, therefore, it can act only as an oxidizing agent by accepting
one or more electrons e.g.
Here, the oxidation number of N decreases from +5 in HNO₃ to +4 in NO ₂ and hence it acts as an oxidizing agent.
2. The oxidation number of S in H₂S is least i.e. -2 and hence it can act only as a reducing agent by losing one or more electrons. Hence the oxidation number of S increases from -2 in H ₂S to 0 in elemental Sulphur and hence it acts as a reducing agent.
3. The oxidation number of N in HNO₂ is intermediate (+3), it is neither maximum (+5) nor minimum (-3), therefore, it can act both as an oxidizing as well as a reducing agent. E.g.
Here, the oxidation number of N increases from +3 in HNO₂ to +5 in HNO₃, therefore, it acts a reducing agent.
Here, the oxidation number of N decreases from +3 in HNO₂ to +2 in NO, therefore, it acts as a an oxidizing agent.
Factors Affecting Strength of Oxidizing and Reducing Agent
1. A substance can act as oxidizing agent if the oxidation number
of one of its element is maximum.
e.g.
HNO₃ in which O.N of N is +5 which is its maximum oxidation state is a strong oxidizing agent.
2. The more the electronegativity of central element and the more
is its oxidation number, the more is the oxidizing power.
e.g.
KClO₄4, KBrO₄, HClO₄, KMnO₄4, K₂Cr₂O₇, HNO3, H₂SO₄ etc.
3. Oxyanions are stronger oxidizing agents in acidic solution than in basic or neutral solution.
4. A substance can act as reducing agent if the oxidation number of
one of its element is minimum.
e.g. SnCl₂ (O.N of Sn =+2 which is least), FeSO₄ (O.N of Fe =+2), Na₂S₂O₃ (O.N of S =+2), H2S
(O.N of S = −2), H₂C₂O₄ (O.N of C =+3) etc.
5. Anions of electronegative elements like I-, Br−, N3−
are powerful reducing agents.
6. A substance that can act as both reducing as well as oxidizing
agent if oxidation number of one its element is in between its maximum and the
minimum oxidation number value.
e.g.
HNO2 (O.N of N =+3 which is intermediate of +5 and 0).
Oxidation number and Acid
Th greater the oxidation number of the central element,
the greater is the acid strength.
HClO4 (+7)
> HClO3 (+75) > HClO2
(+3) > HClO (+1)
Some Oxidizing agent and Reducing Agent
Types of Chemical Reactions According to Electron Transfer
There are three types of chemical reactions based on oxidation and reduction:
1. Non- Redox
Reaction
2. Redox
Reaction or Oxidation-Reduction Reaction (ORR)
3. Auto Redox
Reaction or Self Oxidation-Reduction Reaction (ARR)
Non- Redox Reaction
Definition of Non-Redox Reactions
The chemical reaction in which there is no electron transfer i.e. no substance is oxidized or reduced not undergoing change in oxidation number is called non-redox reaction.
General Examples of Non-Redox Reactions
1. Neutralization
2. Hydrolysis
3. Precipitation
reactions
4. Acid displacement reactions
5. Base
Displacement Reactions
6. Some decomposition reactions
7. All double
decomposition reactions
8. Some molecular addition reactions
Oxidation-Reduction Reactions (ORR) or Redox Reactions
Definition of Oxidation-Reduction (Redox) Reaction (ORR)
Redox reactions are also called electron-transfer reactions since electrons are transferred from the reductant to oxidant.
Oxidation and reduction always occur simultaneously during
a chemical reaction. The chemical reactions in which oxidation and reduction
occur simultaneously are called oxidation-reduction reactions (ORR) or redox
reactions. In other words, these are the reactions in which increase and
decrease in oxidation number of same or different atoms occurs.
These reactions comprising of
simultaneous oxidation and reduction.
In terms of electron transfer, a redox reaction is defined as the process
in which electrons are transferred from one
substance (reducing agent) to another (oxidizing agent).
Explanation
All oxidation and reduction reactions are complimentary of one
another and occur simultaneously, one cannot take place without the other. No
single oxidation and no single
reduction process are known. Oxidation-reduction reactions involve two opposing but complementary processes. These
processes can never occur singly i.e. every oxidation must necessarily be
accompanied by its opposing process reduction and vice versa. The simultaneously oxidation and
reduction reactions are generally termed as redox reactions. The substance which brings reduction is
known as reducing agent while a substance which brings oxidation is known as
oxidizing agent.
Daily life examples of Redox reactions
The reactions taking place in batteries are redox reactions. Redox
reactions take place in the batteries such
that electrons transferred can pass through some external circuit so that they
produce electric current
Digestion and metabolism of food which takes place in our body in order
to supply us the energy required to perform work is also takes place through
a series of redox reactions.
Ordinary bleach oxidize the substances that stain fabric, this makes them colourless and easier to remove from fabric.
redox couple
A redox couple is defined as having together the oxidized and reduced forms of a substance taking part in an oxidation or reduction half reaction. Represented as Zn2+/Zn and Cu2+/Cu.
Example No. 1 of Redox Reaction
The addition reaction between H2 gas and Br2 to
form hydrogen bromide is an example of redox reaction. In this
reaction H2 has been oxidized because its oxidation number
has been increased so H2 is a reducing agent while Br2
has been reduced because its oxidation number has been decreased so Br2
is an oxidizing agent. Thus it is a Redox Reaction during which oxidation
and reduction takes place simultaneously.
Following are the examples of Redox reactions:
Types of Redox Reactions
Redox
reactions are divided into two main types.
(i) Inter
molecular Redox Reactions
(ii) Intra
molecular Redox Reactions
(iii)Disproportionation
(iv) Comproportionatin
reaction (reverse of Disproportionation; HClO + Cl‒ → Cl2
+ OH‒)
Inter molecular Redox Reactions
In such redox reactions, one
molecule of reactant is oxidized whereas molecule of other reactant is reduced.
In this case, one substance is oxidized and another is reduced. In following
reaction, HCl is oxidized while MnO2 is reduced.
(ii) Intra molecular Redox Reactions
In such redox reactions, one
atom of a molecule is oxidized and other atom of same molecule is reduced. In
this case, one element of the compound is reduced while another element of the
same compound is oxidized.
Examples
In the decomposition of KClO3, its Cl is reduced to KCl and O is oxidized to O2.
In the decomposition of (NH4)2Cr2O7, its Cr is reduced to Cr2O3 and N is oxidized is oxidized to N2.
Disproportionation Reaction/Auto-Redox reaction/Self-Redox
reactions
Definition
It is an important and
special type of redox reaction in which a single substance
(specie) undergoes simultaneous oxidation and
reduction i.e. it occurs when a same element is both oxidized
and reduced simultaneously (i.e. in the meantime). A specie undergoing auto-redox reaction is said to be
disproportionate.
disproportionation, also
called disputation reaction, is basically a redox
reaction involving simultaneous reduction and oxidation of atoms of the
same element of a substance of intermediate oxidation state from one
oxidation state to two different oxidation states forming two compounds, one
with higher and one with lower oxidation states. So a species is simultaneously
reduced and oxidized to form two different products.
Example
The requirement for disproportionation reaction to
occur is, the element undergoing disproportionation should exhibit minimum
three different oxidation states and the element must be less stable in a
particular oxidation state from which it can be both oxidized as well as
reduced to relatively more stable oxidation states.
Examples of Auto-Redox Reactions
1. Decomposition or Disproportionation of potassium chlorate to potassium perchlorate and potassium chloride
2. Decomposition of nitrogen (III) oxide into nitric oxide and
nitrogen dioxide
3. Decomposition of hydrogen peroxide into water and oxygen
Decomposition reaction of hydrogen peroxide into water and oxygen involves disproportionation of oxygen. In this auto-redox reaction, the relatively less stable oxygen of peroxide in the -1 oxidation state disproportionates into relatively more stable compounds i.e. water and dioxygen changing its oxidation state to the -2 oxidation state in water and zero oxidation state in oxygen gas at the same time.
4. Dissolution of chlorine gas in water (Reaction of chlorine
gas with water)
5. Photolysis of Mercurous chloride into mercuric
chloride and mercury
Upon UV-irradiation, Mercurous chloride or mercury(I)
chloride undergoes disproportionation. under UV light to give mercury and
mercuric chloride. The Hg22+ ion is oxidized to Hg2+ and
reduced to Hg.
6. Dissolution of nitrogen dioxide in water
When nitrogen dioxide in which oxidation state of nitrogen is +5 reacts with water (Ostwald process), it undergoes disproportionation reaction resulting in the formation of both nitric acid and nitrous acid (or nitric oxide; O.S of N = +2) wherein nitrogen has oxidation states +5 and +3 respectively. In this reaction, nitrogen of NO2 with +4 oxidation state is simultaneously oxidized to nitric acid (+5 oxidation state) and reduced to nitrous acid or NO (with oxidation state +3 or +2). Thus, it is a disproportionation reaction.
7. Decomposition of Cuprous chloride into cupric chloride and copper
Decomposition of Cuprous chloride into cupric chloride
and copper involves disproportionation of copper. When cuprous chloride in
which oxidation state of copper is +1 is heated it is decomposed and
simultaneously oxidized to copper chloride changing the oxidation state of
copper from +1 to +2 and reduced to elemental copper changing the oxidation
state of copper from +1 to 0. Thus, this is a disproportionation reaction.
8. Dissolution of metal superoxides with water
This reaction can serve as a convenient source of oxygen in masks of self-contained breathing apparatus worn by fire fighters. The source of oxygen is the reaction between KO2 and exhaled water vapours. The KOH so formed serves to remove CO2 from the exhaled breath.
9. disproportionation of Phosphorus to phosphine and hypophosphite in alkaline medium.
Phosphorus disproportionates to phosphine and hypophosphite in alkaline medium. In this case, one P atom is reduced to -3 oxidation number (in PH3) and three P atoms get oxidized to +1 (in NaH2PO2).
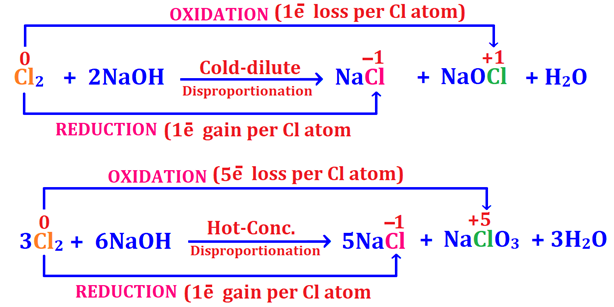
10. Auto-redox Reactions of chlorine gas with dilute or conc Alkalis (sodium hydroxide & lime water)
Chlorine undergoes auto-redox reaction with water, sodium hydroxide (cold and hot) and lime water (cold, hot and dry) in which it reduces to chloride (Cl-) ion (in HCl or NaCl or CaCl2) as well as oxidizes itself to Cl+1 (in hypochlorite; ClO1-) or Cl+5 (in chlorate; ClO31-).
11. Cannizaro’s reaction/Auto-redox Reactions of formaldehyde with conc. Alkalis
The self-addition oxidation
reduction and disproportionation Reaction in which two molecules of aldehyde
lacking a-hydrogen are disproportinated into
carboxylic acid (which form salt with alkali) and alcohol is known as
Cannizaro’s Reaction.
Aldehydes lacking a-hydrogen like formaldehyde and
benzaldehyde (do not show aldol condensation) undergo self-redox reaction in
presence of aqueous alkali, two molecules of such aldehydes disproportionate
and simultaneously oxidize and reduce one another into acid and alcohol respectively.
For example
formaldehyde on heating with conc.
Solution of strong alkali like NaOH undergoes self-oxidation reduction reaction
in one molecule of formaldehyde is reduced to methanol and the other is
oxidized to formic acid that forms salt with alkali.
Comproportionation reaction /synproportionation (opposite of the disproportionation)
Comproportionation reaction is the
opposite of disproportionation reaction. In this reaction, two reactants with
the same element in different oxidation states combine to form the same element
in the intermediate oxidation state.
The reverse of disproportionation,
such as when a compound in an intermediate oxidation state is formed from
precursors of lower and higher oxidation states, is called comproportionation,
Ag2+(aq) + Ag(s)
→ 2Ag+(aq)
Oxidation Number (O.N)
OR Oxidation State (Oxi. No.)
Definition of Oxidation Number
In covalent bond formation the electrons
are not transferred as in ionic bond formation, but partial
transfer of electronic charge takes place, known as electron shift. The oxidation
number method always assumes that there is a
complete transfer of electrons from a less electronegative atom to a more
electronegative atom.
Oxidation Number is a fictitious charges assigned to the atom of an element
in a covalently bonded molecule by arbitrary
conventions that results when the electrons in
a covalent bond are assigned to the more electronegative atom (assuming the
bonding were ionic) making certain the law of charge conservation is strictly
obeyed. Oxidation Number is a purely a hypothetical number without any theoretical
justification and it does not correspond (coincide with) to the real (actual)
charge on the atoms, except in the special case of simple ionic compounds. Oxidation
number of an atom in a molecule or ion is the hypothetical or
real charge present on an atom due to electronegativity
difference.
the number of charges an atom
would have in a molecule of a compound or polyatomic ion if bonding electrons
were transferred completely in the direction indicated by the difference in
electronegativity. Thus oxidation number reflects the number of electrons
transferred in a covalent molecule or polyatomic ion. It is the number of electrons lost
or gained by an atom of an element during its change from free state into a
particular compound.
OR
the apparent charge (i.e. not
real), either positive or negative or zero, on an atom of element in a molecule
of a compound or in a polyatomic ion (radical) that results when the electrons
in a covalent bond are assigned to the more electronegative atom is called
oxidation number or oxidation state. Its value may be positive, negative or zero even
fractional value ranges -4 to +7 (+8) depending upon the charge of combined
atoms in the molecule or ion. (In Ni (CO)4 oxidation number of Ni is
zero).
OR
It is the fictitious
charge that an atom appears to have in a given species when the bonding
electrons are counted towards more electronegative atom i.e. it is the hypothetical charge an atom would
possess in a compound if the bonding were completely ionic.
Oxidation number as the degree of Oxidation
Oxidation number is the number with positive or negative sign which
indicates the extent to which an element has been oxidized or reduced i.e. it
shows the number of electrons which an atom has lost or gained as a result of
bonding. The oxidation state is a “measure (or indicator) of the degree of
oxidation” of an atom in a chemical compound. (In writing oxidation numbers, we
will write the sign before the number to distinguish them from actual
electronic charges, which we write with number first). It is the fundamental
key to understanding redox reactions, reaction mechanisms, catalysis, etc.
Basis
of Assigning Oxidation Number
The
oxidation number for an element in a covalent compound is by taking the
oxidation number to be equal to the charge that the element would carry, if all
the bonds in the compound were regarded as ionic instead of covalent. In doing
this, a shared pair or electrons between two atoms is assigned to the atom with
the greater electronegativity. Or, if the two atoms are alike, the shared pair
is split between the two, one electron being assigned to each atom. The resulting
charges on the various atoms when the bonding electrons are so assigned are the
oxidation numbers of the atoms.
Covalency
It is the
number of hydrogen atoms which can combine with a given atom. It is equal to
the number of single bonds which an atom can form. It is also equal to the
number of electrons an atom can share.
Oxidation State
It is the oxidation number per
atom.
Examples of Oxidation Number
1. Oxidation number of Mn in KMnO4 is +7.
2. Oxidation number of Cr in K2Cr2O7 is +6.
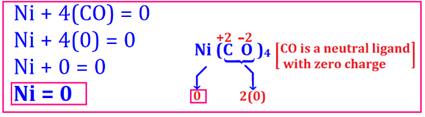
3. Oxidation number of Ni in Ni(CO)4 is 0.
4. Oxidation number of O in OF2 is +2.
5. Oxidation number of O in KO2 is –½.
6. Oxidation number of O in H2O2 is –1.
Difference between Valency and Oxidation number
Valency is a different term than oxidation number though sometimes the
valency and the oxidation number of an element are same in a compound.
1. oxidation number is just the apparent charge (not necessarily actual) over
the atom when the electrons are counted according to the arbitrary rules i.e.
oxidation number is the number with positive or negative sign which indicates
the extent to which an element has been oxidized or reduced. While valency is
mere a number without positive or negative sign which expresses the combining
or displacing tendency of an atom of an element and valency of an element is
given by the number of electrons it actually loses or gains or shares during
the formation of a compound.
2. The oxidation no. of an atom may be in fraction, whereas the valency
is always in whole number. The oxidation number of an atom in a compound may be
zero but valency of an element cannot be zero (except noble gases).
3. the oxidation state of
an element may vary in its different compounds whereas in most of the cases,
the valency of an element is constant.
4. Valency and oxidation states of carbon in its different compounds give
a good example to differentiate the two concepts. In CH4, CH3Cl,
CH2Cl2, CHCl3 and CCl4, the valency
of carbon is always four (due to sharing of four electrons) but its oxidation numbers is -4, -2, 0, +2 and +4 respectively.
Oxidation State Vs Valency
Some Important Points on Oxidation Number
1. Oxidation number may be fractional.
2. Oxidation number is positive in metallic elements
3. Oxidation number is positive or negative in
non-metallic elements
4. Oxidation
number is represented in Roman numbers in
parenthesis (brackets) after the symbol of the
metal in compounds
Stock Notation
Representation of oxidation state of element by Roman numerals within
parenthesis is known as stock notation i.e. Expressing the oxidation state of a
metal by Roman numerals like I, II, III etc. within parenthesis is called stock
notation.
e.g.
Fe(II) SO4 or FeSO4 = Iron(II) sulphates (or ferrous sulphate)
Fe(III) or FeCl3 = iron(III) chloride (or ferric chloride)
Au(III) Cl3 or AuCl3 = Gold(III) chloride (or auric chloride)
Sn(II) Cl2 or Sn(II) Cl2 = Tin(II) chloride (or stannous chloride)
Hg(II)Cl2 or Hg(II)Cl2 = Mercury(II) chloride (or mercuric chloride)
Na2CrO4 = sodium chromate(VI)
5.The oxidation
number of metals in amalgams and metal carbonyls i.e. Ni(CO)4, Fe(CO)3, Cr(CO)6 etc. is zero.
6. In
allotropic forms like diamond, graphite etc.
oxidation number is 0.
7. In case of coordinate bond, it gives +2 value of oxidation number to less electronegative atom and -2 values to more electronegative atom when coordinate bond is directed form less electronegative atom to more electronegative atom.
8. If coordinate bond is directed from more electronegative to less electronegative atom then its contribution be zero for both the atoms.
9. Oxidation number of O in compounds of fluorine is positive as F is the most electronegative element.
10. Electronegativity values of no two elements
are same
P > H, C > H, S > C, Cl > N
11. Oxidation state of same element
can be different in same or
different compounds
O.N of S in H2S = ‒ 2
O.N of S in H2SO3 = +4
O.N of S in H2SO4 = +6
Details of Important Points of Oxidation Number
1. Positive and
Negative Oxidation Numbers
2. Fractional Oxidation Number
3. Range of
Oxidation Number
4. Maximum Oxidation
Numbers
5. Oxidation Number measures covalent and ionic
character
7. Oxidation number and group number
1. Positive and Negative Oxidation Numbers
In general metallic elements (present at the farther left and middle of the periodic table) have only positive oxidation
numbers (e.g. Li, Na,
K, Mg, Ca, Ba, Al, Pb, Sn, Fe, Cu, etc.) whereas non-metallic elements may have either positive or negative oxidation numbers. But usually non-metals such as F,
O, N and other halogens (Cl, Br, and I) have negative oxidation numbers.
2. Fractional Oxidation Number
Elements as such
do not have any fractional oxidation numbers. In reality no
element can have a fractional oxidation state as electrons cannot be
transferred in fraction.
When two or
more atoms of an element are present in different oxidation states, then
calculated oxidation number in a compound or ion may come out as fractional due
to average of all the different oxidation
states.
Fractional
oxidation number is the average oxidation number. Fractional oxidation number
of a particular element can be calculated only if we know about the structure
of the compound in which it is present.
e.g.
(i) In tetrathionate
(S4O62−) ion, the oxidation number of end S atoms is +5 each and that of
the middle S atoms is 0 each. The total oxidation
number of 4 S atoms is 5+0+0+5=+10 and the average
oxidation number is 10 ÷ 4 = 2.5.
(ii) In sodium tetrathionate (Na2S4O6),
the oxidation number of both S+ is equal to 0 (pure covalent bond)
and other two terminal sulphur atoms have oxidation number = +5.
having the structure
(iii) in C3O2 the
oxidation number of C is +4/3 or + 1.33.
3. Range of
Oxidation Number
Oxidation number of an atom in a molecule may be positive, negative or
any value ranges –4 to zero to +7 (or +8 in Os+8O4, Ru+8O4
etc. or even fractional value
e.g. Fe3O4 (Fe = +2.6), C3O4 (C = +2.6), C3O2 (C = +1.33). Oxidation number of an atom in a molecule may have zero value. e.g. Ni(CO)4 (Ni = 0), Fe(CO)5 (Fe=0), C6H12O6 (C=0), C12H22O11 (C =0), CH2O (C =0) etc.
The highest known oxidation state is +8 in the tetroxides of ruthenium,
xenon, osmium, iridium, hassium, and some complexes involving plutonium; the lowest known oxidation state is −4 for some elements in the carbon group.
4. Variable
Oxidation Numbers
The transition metals of group B of the periodic table usually have several possible oxidation states except IIIB
group (Sc, Y, La and Ac = +3) and IIB group (Zn, Cd = +2). The variable oxidation state of d-block elements is due to
involvement of unpaired electrons of d-subshell. Similarly p-block elements
exhibit variable oxidation states which is due to inert pair effect.
5. Maximum
Oxidation Numbers
Maximum oxidation number is always positive and maximum
oxidation numbers of an atom in a molecule is equal to its group number. e.g. maximum oxidation number of Cl of group VIIA is +7 and
that of Cr of group VIB is +6.
▶ Os, Ru, Xe show maximum oxidation number i.e. +8
▶ In 3d-series of transition metals, Mn shows maximum oxidation number of
+7.
▶ Maximum oxidation number of element = Group number in periodic table
▶ Minimum oxidation number of element = Group number – 8 (applicable only
for non-metals).
Maximum or highest oxidation state is not stable. (Thus compounds containing central
atom with its highest oxidation state are unstable and tend to decompose to
reduce oxidation number
e.g. KMnO4 (Mn = +7), AgNO3 (N = +5), Mn2O7
(Mn = +7), HClO4 (Cl = +7) etc.
▶ For
p-block elements (except F and O), the highest oxidation number is equal to
their group number and lowest oxidation number is equal to the group number minus eight.
▶ In
transition elements the lowest oxidation number is equal to the number of ns
electrons and highest oxidation number is equal to number of ‘ns’ and (n–1)d
unpaired electrons. Maximum oxidation
number of transition elements is given by:
Maximum
oxidation number of atom = Number of ‘s’ electrons + Number of unpaired ‘d’
electrons
e.g.
Maximum oxidation number of Mn = 2
+ 5 = +7
Maximum oxidation number of Fe = 2 + 4 = +6
6. Oxidation
Number measures covalent and ionic character
A high
oxidation number usually
indicates significant
(more) covalent character in the bonding of that compound
e.g. Mn2O7 (Mn=+7) and MnO4 (Mn=+7) ion
have more covalent character.
Compounds with lower oxidation states have more ionic character
e.g. MnO2 (Mn=+4) and Mn2O3 (Mn=+3) have
significant ionic character.
7. Oxidation number
and group number
Oxidation number is directly related to the group number to which the
element belongs.
E.g. the oxidation number of group IA is +1 and that of IIA is +2. Similarly zero group shows zero oxidation state.
Oxidation number of p-block elements is the number of electrons in the
valence shell or deficiency of electrons in the valence shell.
8. Oxidation number of two or more atoms of same elements may be different
If a compound contains two or more atoms of the same
element, all of them may or may not have same oxidation number
e.g.
(i) In Na2S2O3,
one S-atom has oxidation number = -2 while the other has oxidation number = +6.
(ii) In bleaching powder; CaOCl2 or
Ca(OCl)Cl, oxidation number of one Cl = ‒1 while oxidation number of other Cl = +1.
(iii) In Fe3O4 or FeO.Fe2O3,
oxidaiton number of one Fe = +2 while that of each of the other two = +3.
(iv) In NH4NO3, oxidation number
of N of NH4+ = ‒3 while that of N in NO3‒
= +5.
How to get Oxidation
Number
1) Given a
compound, write its Lewis dot structure.
2) For each
separate bond decide which element is most electronegative (EN).
3) Give the most electronegative element all the electrons
of that bond. If the atoms in the bond are the same, give each element half of
the electrons.
4) When all electrons have been assigned subtract the
number of electrons on each atom from the valence of each element to get the oxidation
state (number).
Significance of Oxidation number
Oxidation number provides a measure of whether the atom in a molecule is
neutral, electron rich or electron-poor. It guides us to identify elements that
are oxidized (oxidation number increases) and reduced (oxidation number
decreases) at a glance by comparing its oxidation number before and after the
reaction.
Oxidation numbers are used:
1. In nomenclature (naming) of
compounds.
2. In classifying
types of reactions (as redox, non-redox
or auto-redox).
3. In balancing of equations
of redox reactions.
4. In examining
trends in chemical reactivity across the
periodic table.
5. In exploring
the systematic chemistry of elements.
6. In identifying
redox (oxidation-reduction) reactions.
7. In determining
the Equivalent weights
8. in
comparing
the strength of acid and base
(a) Strength
of acids increases with increase
in oxidation number.
(b) Strength
of base decreases with increase
in oxidation number.
9. In determining the oxidizing and reducing nature of
compounds
(a) If any
compound is in maximum oxidation state, then it will act as oxidant only.
(b) If any
compound is in minimum oxidation state, then it will act as reductant only.
(c) If the oxidation state is intermediate, then compound can act as both reductant as well
as oxidant.
10. To determine possible molecular formula of any compound
Suppose that there are three atoms A, B, C and their oxidation number are
+6, ‒1, ‒2
respectively. Then the molecular formula of compound formed by them will be AB4C
because
+6 = (‒1
or +6 = ‒6
details
in comparing
the strength of acid and base
Rules for
Finding Oxidation Number
1. Oxidation Number
of Free Elements is zero
The oxidation number of an atom in its elemental form or uncombined state is always zero i.e. the oxidation number of an element in a free atomic
state (Na, H, Cl, O,
P etc.) or in its poly-atomic
state (graphite, H2, O2, P4, S8
etc.) or alloy form (Na/Hg) is
always zero. (Oxidation number is zero for any elemental substance, which occurs in
diatomic, triatomic or polyatomic forms or allotropic forms (diamond, graphite)
or alloy form (Na/Hg). (Free state = most stable state, uncombined state).
e.g. each atom in Na, Mg, C, O2,
N2, H2, Br2, F2, I2, O3,
P4, S8, has an oxidation number of zero.
(i) Oxidation state of atoms present in homoatomic molecules is zero.
e.g. H2, O2, N2, P4, S8
= zero
(ii) Oxidation state of an element in any of its allotropic form is zero.
Cdiamond = 0, Cgraphite = 0, Smonoclinic = 0, Srhombic = 0
(iii) Oxidation state of all the components of any an alloy are 0 e.g. Na0/Hg0
(iv) In complex compounds, oxidation state of some neutral ligands is
zero. e.g. CO, NO, H2O, NH3
2. Oxidation Number of Monoatomic Ion equal to
its charge
The oxidation number of atom in
monoatomic ion (composed of only one atom) is equal to its charge.
e.g.
oxidation number of Na in Na1+
is +1, that of Ba in Ba2 + ion is +2, that of Al in Al3+
ion is +3, that of Ca in Ca2+ is +2, that of Cl in Cl– ion is – 1, that of O
in O2– ion is – 2, that of P
in P3– ion is – 3 and so on.
3. Oxidation Number
of atoms in Polyatomic Ion
The oxidation number an atom in a
polyatomic ion is usually equal to its oxidation number that it would have if
it were a monoatomic ion.
For example;
in hydroxide ion (OH–),
the oxygen atom has an oxidation number of –2 as if it were a monatomic oxide
(O2–) ion and the hydrogen atom has an oxidation number of +1 as if
it were simple H+ ion.
[In oxyanions, the oxidation number
of central atom is always positive which is usually equal to its highest
oxidation number e.g. in CO32– ion, the oxidation number
of carbon is +4 which is its highest oxidation state].
4. Sum of Oxidation
Numbers of all atoms in Polyatomic Ion equals net ionic charge
In polyatomic ions (or compound
radical), the sum of oxidation numbers of its all atoms is equal to overall
(net) charge of the ion.
e.g.
in the ammonium ion (NH4+),
the oxidation number of each H is +1 and that of N is –3. Thus the sum of the
oxidation numbers is –3 + 4(+1) = +1, which is equal to net charge of the ion.
5. Sum of Oxidation
Numbers of all atoms in a molecule is always zero
In a neutral species (molecule of a compound), the sum of the oxidation numbers of all elements is always zero to comply with law of charge conservation.
e.g.
[This rule is particularly useful
for finding the oxidation number of an atom in difficult cases by assigning
oxidation numbers to the ‘Easy’ atoms first and then find the oxidation number
of the ‘Difficult‘ atom by subtraction].
6. Oxidation Number
of Atoms in Binary Polar Compounds
In binary polar compounds (those with two different elements), more electronegative element has negative oxidation number (equal to its charge in simple ionic compounds of the element) while less electronegative element has positive oxidation number.
e.g.
7. Oxidation Number of Atoms in Ternary Compounds
In ternary polar compounds (those with three or more different elements), only more electronegative element has negative oxidation number (equal to its normal oxidation number) while all other elements have positive oxidation numbers.
8. Oxidation Number of Fluorine is always –1
The oxidation number of Fluorine in
its compounds is always –1.
(Due to
restriction of negative oxidation number, F cannot form oxyacids or oxyanions
for that it has to assign positive oxidation number).
9. Oxidation Number of Other Halogens is usually –1
The oxidation
number of other halogens (Cl, Br and I) in binary compounds where they occur as
halide ion (X‒) is usually
–1. e.g.
The major exception is in compounds
or ions of Cl, Br and I where they are bonded to oxygen atom e.g. in oxyacids,
oxysalts and oxyanions of halogens like H+1Cl+7O4–8,
Na+1Cl+5O3–6, Cl+1O1–
etc.
10. Oxidation
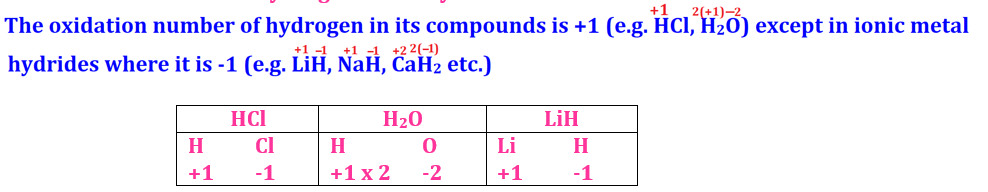
Number of Hydrogen is mostly +1
11. Oxidation
Number of Oxygen
The oxidation number of oxygen in most of its compounds is usually
–2 (e.g. MgO–2, Na2O–2,
P2O5–2, NO–2, Cl2O–2,
SO2–2 etc.). However in
12. Oxidation
Number of Elements in Groups of the Periodic Table
The oxidation number of each
element of Group IA (Li, Na, K, Rb, Cs), IIA (Be, Mg, Ca, Sr, Ba), IIIA (B,Al),
IVA (C, Si, Ge, Sn, Pb), VA (N, P, As, Sb, Bi) and VIA (O, S, Se, Te), in their
compounds is +1, +2, +3, –4/+4, –3/+5, –2, –1, +1 to +6 respectively. (The most
common oxidation state of group IIIA is +3 but it can also show +1 oxidation
state due to inert pair effect).
Some helping rules for calculating oxidation number.
Q1.
Determine the oxidation number of central atom in following
(i) S in Na2S2O3
(ii) Mn in MnO4−
(iii) Cr in Cr2O72−
(iv) Cl in ClO3
(v) Cr in Cr2(SO4)3
(vi) P in Ca(H2PO4)2
(vii) S in H2SO4
(viii) Ni in Ni(CO)4
(ix)
Fe in Fe3O4
(x) C in C3O4
(xi) Fe in Fe(CO)3
(xii)
Cr in Cr(CO)6
Solution
(i)
Finding out Oxidation number of S in Na2S2O3
Oxidation
number of Na = +1
Oxidation number of O = −2
Na2S2O3
is a neutral compound in which sum of the oxidation numbers of all atoms is
zero.
2(Na) + 2(S)
+ 3(O) = 0
2(+1) +
2S + 3(−2) = 0
+2 + 2S +
(−6) = 0
2S + −4 =
0
2S = +4
S = +4/2 = +2
In Na2S2O3, one S-atom (S*) has
oxidation number = −2 while the other S-atom (S**) has
oxidation number = +6. The total oxidation number of both S atoms = −2 + +6 = +4
The average oxidation number of S = Total Oxidation number/2
= +4/2 = +2
(ii)
Finding out Oxidation number of Mn in MnO4−
Oxidation number of O = −2
MnO4−
is a polyatomic ion in which sum of the oxidation numbers of all elements is
equal to its net ionic charge.
Mn + 4(O)
= −1
Mn +
4(−2) = −1
Mn −8 =
−1
Mn = −1 +
8
Mn
= +7
(iii)
Finding out Oxidation number of Cr in Cr2O72−
Oxidation number of O = −2
Cr2O72−
is a polyatomic ion in which sum of the oxidation numbers of all elements
is equal to its net ionic charge.
2Cr +
7(O) = −2
2Cr +
7(−2) = −2
2Cr −14 =
−2
2Cr = −2
+ 14
2Cr = +12
Cr =
+12/2 = +6
(iv)
Finding out Oxidation number of Cl in ClO3
Oxidation number of O = −2
ClO3
is a neutral compound in which sum of the oxidation numbers of all atoms is
zero.
Cl + 3(O) = 0
⇒ Cl + 3(−2) = 0
⇒ Cl −6 = 0
⇒ Cl = +6
(v)
Finding out Oxidation number of Cr in Cr2(SO4)3
Oxidation number of SO4 = −2
Cr2(SO4)3 is a neutral compound in which sum of the oxidation numbers of all elements is equal to zero.
2Cr +
3(SO4) = 0
2Cr + 3(−2) = 0
2Cr −6 = 0
2Cr = +6
Cr = +6/2 = +3
Alternate
method
In sulphate (SO42−) ion, oxidation number of S is +6.
2Cr +
3(S) + 12(O) = 0
2Cr + 3(+6) + 12(−2) = 0
2Cr + 18 + (−24) = 0
2Cr + 18 −24 = 0
2Cr −6 = 0
2Cr = +6
Cr = +6/2 = +3
(vi)
Finding out Oxidation number of P in Ca(H2PO4)2
Oxidation number of Ca = +2
Oxidation number of H = +1
Oxidation number of O = −2
Ca(H2PO4)2
is a neutral compound in which sum of the oxidation numbers of all
elements is equal to zero.
Ca + 4(H)
+ 2P + 8(O) = 0
+2 +
4(+1) + 2P + 8(−2) = 0
+2 + 4 +
2P + (−16) = 0
+2 + 4 +
2P −16 = 0
2P −10 =
0
2P = +10
P = +10/2
= +5
(vii)
Finding out Oxidation number of S in H2SO4
Oxidation
number of H = +1
Oxidation
number of O = –2
H2SO4
is a neutral compound in which sum of the oxidation numbers of all atoms is
zero.
(viii) Finding out Oxidation number of
Ni in Ni(CO)4
The oxidation number of metals in
amalgams and metal carbonyls i.e. Ni(CO)4, etc. is zero. In fact, in
this transition metal complex, CO (called carbonyl) is a neutral ligand with
overall charge of zero, hence giving zero oxidation number to central atom Ni.
Some other neutral ligand is NO, H2O, NH3, PH3,
en, etc.
The oxidation number may be
calculated as
(ix)
Finding out Oxidation number of Fe in Fe3O4
Fe3O4 is a
mixed oxide consisting of two oxides ferrous oxide; FeO (O.N of Fe =+2)and
ferric oxide, Fe2O3 (O.N of Fe =+3). The average
oxidation of Fe is +8/3 or +2.66.
Oxidation
number of Fe in FeO = +2
Oxidation number of Fe in Fe2O3
= +3
Average oxidation number = 2+
2(+3)/2 = +8/3
The oxidation number may be
calculated as
3(Fe) + 4(O) = 0
3Fe + 4(−2) = 0
3Fe + (−8) = 0
3Fe = +8
Fe = +8/3 or + 2.66
(x) Finding out Oxidation number of C in C3O4
3C + 4(O) = 0
3C + 4(−2) = 0
3C + (−8) = 0
3C= +8
C= +8/3 or + 2.66
(xi) Finding out Oxidation
number of Fe in Fe(CO)3
The
oxidation number of metals in amalgams and metal carbonyls i.e. Fe(CO)3
, Ni(CO)4, Cr(CO)6 etc. is zero. In fact, in this
transition metal complex, CO (called carbonyl) is a neutral ligand with overall
charge of zero, hence giving zero oxidation number to central atom Fe. Some
other neutral ligand is NO, H2O, NH3, PH3, en,
etc.
The oxidation number may be
calculated as
Fe + 3(CO) = 0
Fe + 3(0) = 0
Fe + 0 = 0
Fe = 0
(xii) Finding out Oxidation number of Cr in Cr(CO)6
The
oxidation number of metals in amalgams and metal carbonyls i.e. Fe(CO)3
, Ni(CO)4, Cr(CO)6 etc. is zero. In fact, in this
transition metal complex, CO (called carbonyl) is a neutral ligand with overall
charge of zero, hence giving zero oxidation number to central atom Cr. Some
other neutral ligand is NO, H2O, NH3, PH3, en,
etc.
The oxidation number may be calculated as
Cr+ 6(CO) = 0
Cr + 6(0) = 0
Cr = 0
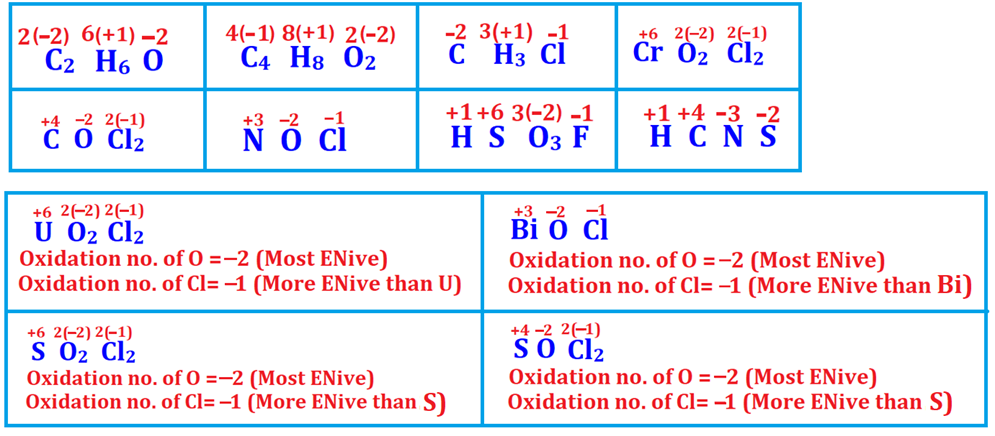
Q2. Calculate the oxidation number of central element in following:
(i) C in C2H6O
(ii) C in C4H8O2
(iii) C in CH3Cl
(iv) Cr in CrO2Cl2
(v) C in COCl2
(vi)
N in NOCl
(vii) U in UO2Cl2
(viii)Bi in BiOCl
(ix) S
in SO2Cl2
(x) S in SOCl2
(xi) S in HSO3F
(xii)
C in HCNS
Solution
Note
In few organic compounds and in few inorganic compounds where
two more electronegative elements are present, more than one element has
negative oxidation number while only one element has positive oxidation number.
Q4. Calculate the oxidation number of central element in following:
(i) P in POCl3
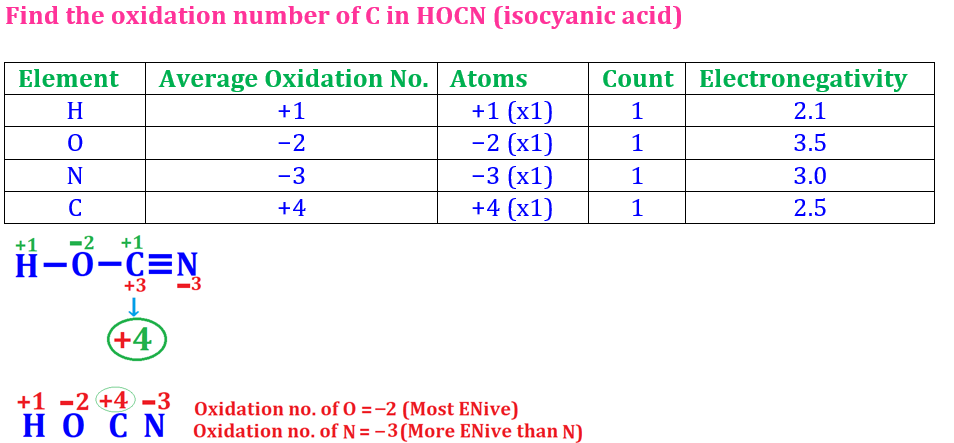
(ii) CNO−
(iii) Cl in CaOCl2 (Bleaching powder)
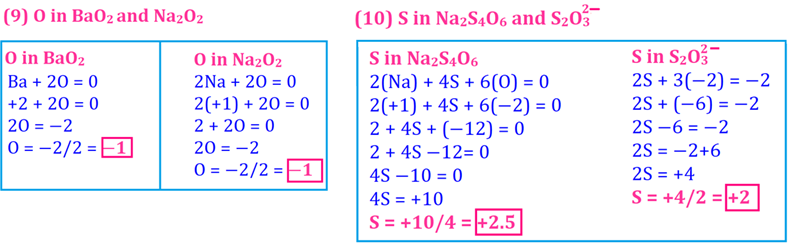
(iv) S in Na2S4O6
(v) N in NH4NO3
(vi) Br in BrO3−
Solution
(i)
Finding out Oxidation number of P in POCl3
Oxidation number of Cl = −1 (since Cl is
more electronegative than P)
Oxidation number of O = −2 (Normal oxidation number of O)
POCl3
is a neutral compound in which sum of the oxidation numbers of all atoms
is zero.
(ii)
Finding out Oxidation number of C in CNO−
Oxidation number of N = −2 (since N is
more electronegative than C)
Oxidation number of O = −2 (Normal
oxidation number of O)
CNO−
is a polyatomic ion in which sum of the oxidation numbers of all elements is
equal to its net ionic charge.
(iii)
Finding out Oxidation number of Cl in CaOCl2
(Bleaching powder)
CaOCl2 (Bleaching powder) is a mixed salt
containing two anions namely chloride (Cl−) ion with −1 oxidation
number of Cl and hypochlorite (OCl−) ion with +1 oxidation state of
Cl.
(iv)
Finding out Oxidation number of S in Na2S4O6
(v)
Finding out Oxidation number of N in NH4NO3
NH4NO3 (ammonium nitrate) consists of
two nitrogen containing ionic species namely ammonium ion (NH4+)
in which oxidation number of N is −3 and nitrate ion (NO3−)
in which oxidation number of N is +5. The total oxidation number of both
nitrogen is +2 and the average oxidation number is +1.
Oxidation number of N in NH4+
N + 4(H)
= +1
N + 4(+1)
= +1
N + 4 =
+1
N = +1 −
4
N =
− 3
Oxidation
number of N in NO3−
N + 3(O)
= −1
N + 3(−2)
= −1
N − 6 =
−1
N = −1 +
6
N =
+5
Average
Oxidation number of N
2(N) +
4(H) + 3(O) = 0
2N +
4(+1) + 3(−2) = 0
2N + (+4)
+ (−6) = 0
2N + −2 =
0
2N = +2
N =
+2/2 = +1
(vi)
Finding out Oxidation number of Br in BrO3−
Oxidation number of O = −2 (Normal
oxidation number of O)
BrO3−
is a
polyatomic ion in which sum of the oxidation numbers of all elements is equal
to its net ionic charge.
Br + 3(O)
= −1
Br +
3(−2) = −1
Br −6 = −1
Br = −1+6
Br
= +5
Q5. Calculate the oxidation number of central element in following:
(i)NO2
(ii) OF2
(iii) NCl3
(iv) CO
(v) BN
(vi) SO3
Solution
Q6.
Calculate
(i)
Oxidation number of S in tetrathionate (S4O62−)
ion
(ii) Oxidation number of C in C3O2
(carbon suboxide)
(iii)
Oxidation number of U in UO2(NO3)2
(iv)Oxidation number of Cr in CrO5
(Perchromate; Blue)
(v)
Oxidation number of Cl in NaClO4
(vi)
Oxidation number of Cr in SrCr2O7
(vii)
Oxidation number of B in Na2B4O7
(vii)
Oxidation number of Br in Br3O8
Solution
(i)
Finding out Oxidation number of S in tetrathionate (S4O62−)
ion
The oxidation number of end S atoms
is +5 each and other two middle sulphur atoms shown by S*
(pure covalent bond) is 0 each. The total oxidation number of 4 S
atoms is 5+0+0+5=10 and the average oxidation number is 10 ÷ 4 = 2.5.
(ii)
Finding out Oxidation number of C in C3O2
In C3O2
(carbon suboxide), the central C-atom has oxidation number = 0 (pure or
non-polar covalent bond) while the other two C-atoms have oxidation number = +2
each.
The total oxidation number of all
the three carbon atoms = +2 + 0 +2 = +4
The average oxidation number of C =
total oxidation number/3 = +4/3
or + 1.33
(iii)
Oxidation number of U in UO2(NO3)2
Oxidation number of O = −2
Oxidation
number of NO3− = −1
U + 2(O)
+ 2(NO3) = 0
U + 2(−2)
+ 2(−1) = 0
U + (−4)
+ (−2) = 0
U −6 = 0
U = +6
(iv)
Oxidation number of Cr in CrO5
(Perchromate; Blue)
The
oxidation number of Cr in CrO5 cannot be calculated by the orthodox
method of computing oxidation number as it is found to be +10 which is greater
than the group number of Cr i.e. VIB and an element cannot exceed its maximum oxidation
number than its group number, so this oxidation number is wrong.
Cr + 5(O) = 0
Cr + 5(−2) = 0
Cr −10 = 0
Cr = +10 (wrong as it is greater than group number of Cr i.e.
VIB)
The oxidation
number of Cr is calculated from its structure
(vii)
Oxidation number of Br in Br3O8
3Br +
8(O) = 0
3Br +
8(−2) = 0
3Br −16 =
0
3Br = +16
Br
= +16/3 or +5.33