Welcome to Inamjazbi Learn Chemistry! 🌟 This post is designed to help Class XII students and MDCAT/ECAT aspirants master Chapter 5–Hydrocarbons with model test questions, practice MCQs, and quick revision tips. Sharpen your concepts, boost your confidence, and ace your exams! 💫
#Hydrocarbons #XIIchemistry #MDCAT #ECAT #OrganicChemistry #ChemistryMCQs #ExamPrep #ModelTestQuestions #InamJazbi #LearnChemistry
🌿🔥 Model Test Questions XII Chemistry | Chapter 5: Hydrocarbons (alkanes, alkenes, alkynes, benzene, phenols) | FSC/MDCAT/ECAT Prep
🌈🔥Short Questions from Alkanes and Cycloalkanes from Textbook
Q1. Alkanes are generally referred as paraffins due to their less reactivity, why are they stable towards chemical reactions.
Q2. Give three differences between aliphatic and aromatic hydrocarbons.
Q3. Define two types of Bond Cleavage or Fission in Organic Chemistry with examples.
Q4. How will you prepare methane or ethane from following?
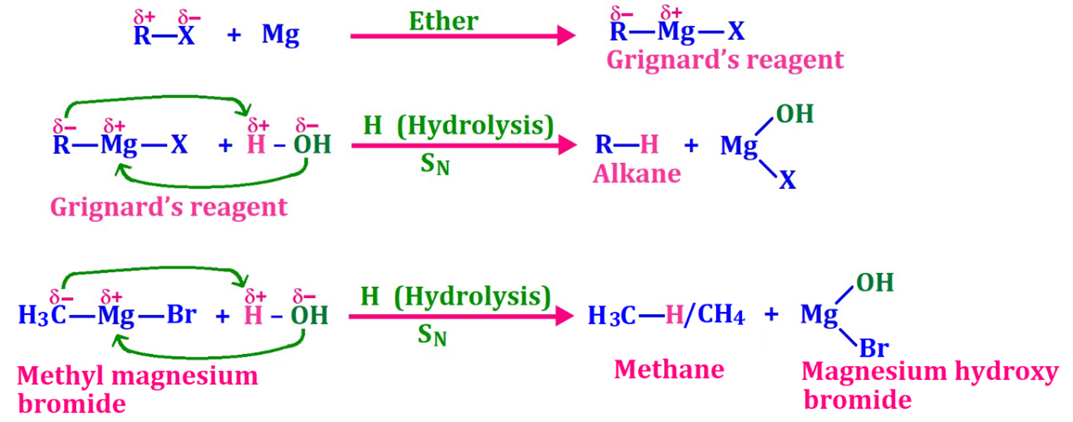
Grignard’s reagent (Hydrolysis), alkanoic acids (decarboxylation), and alkynes
Q5. How will you obtain the following?
(i) Ethane from sodium salt of carboxylic acid [Decarboxylation]
(ii) Ethane from Grignard’s reagent [Hydrolysis]
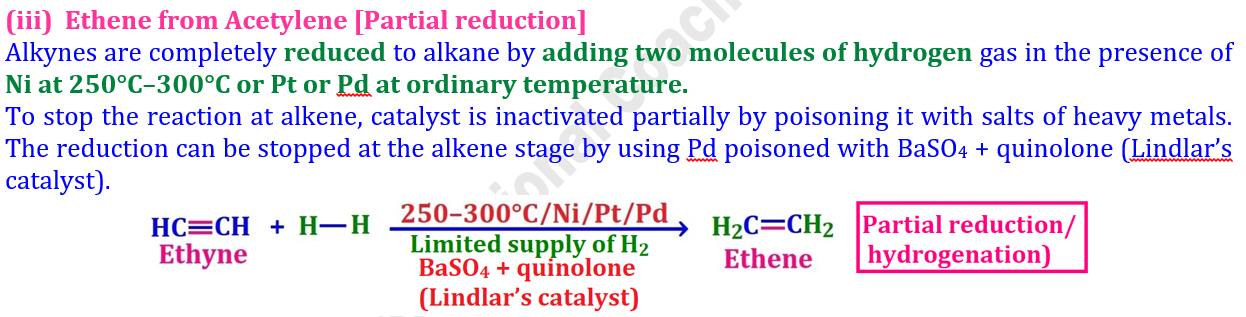
(iii) Ethene from Acetylene [Partial reduction]
(iv) Methane from sodium salt of carboxylic acid [Decarboxylation]
(v) Alkane using Grignard’s reagent and primary amine [Hydrolysis and aminolysis]
Q6. Describe the structure of Cycloalkanes. Why are Cycloalkanes are more stable than alkanes?
🌈🔥Descriptive Questions from Alkanes and Cycloalkanes
Q1. Give the mechanism of free radical reaction between methane and chlorine in the presence of sunlight.
Q2. Describe the preparation of alkane by Reduction of aldehyde and Ketone (Clemmensen reduction and Wolf Kishner reaction).
Q3. Draw the orbital structures of the following hydrocarbons
(i) Ethane (ii) Ethylene (iii) Acetylene
OR
Distinguish between s & p bond. Identify each of them in ethane, ethene & ethyne, by drawing their orbital structures.
🔥📘 Hydrocarbons Rapid-Fire Ultimate Mega MCQs Challenge| High-Yield Practice for Boost XII, MDCAT, ECAT
1️⃣ The carbon atoms in cyclopropane are sp³-hybridized arranged in a …………. geometry
🟦 A. Linear
🟩 B. Tetrahedral
🟧 C. Trigonal
🟪 D. Angular
🟦 A. Linear
🟩 B. Tetrahedral
🟧 C. Trigonal
🟪 D. Angular
2️⃣ Grignard’s Reagent is chemically called
🟩 A. Tetraethyl lead
🟪 B. Methylcobalamine
🟦 C. Alkyl magnesium halide
🟧 D. Dimethyl zinc
3️⃣ Which compound is used as solvent during the synthesis of Grignard’s Reagent?
🟧 A. Alcohol
🟥 B. Water
🟦 C. Ether
🟩 D. Acetic acid
4️⃣ The intermediate formed during Wolff–Kishner reaction is
🟦 A. Hydrazone
🟩 B. Hydrazine
🟧 C. Oxime
🟪 D. Imine
5️⃣ Which of the following reacts most readily with Br₂ (g)?
🟦 A. C₂H₂
🟩 B. C₃H₆
🟧 C. C₂H₄
🟪 D. C₄H₁₀
6️⃣ Reagent used for decarboxylation of sodium alkanoate is
🟦 A. Tollen’s reagent
🟩 B. Soda lime
🟧 C. Soda ash
🟪 D. None of them
7️⃣ The metal used in Grignard’s reagent belongs to group
🟥 A. IA
🟦 B. IIA
🟧 C. IIB
🟩 D. VIIIB
8️⃣ Which one is an organometallic compound?
🟦 A. Grignard’s Reagent
🟩 B. Haemoglobin
🟧 C. Chlorophyll
🟪 D. All of them
9️⃣ Homolytic fission of a neutral molecule gives
🟥 A. One free radical
🟧 B. Two free radicals
🟩 C. Two ions
🟦 D. None
🔟 Wolff–Kishner reduction converts carbonyls to
🟩 A. Hydrocarbons
🟦 B. Alcohols
🟧 C. Acids
🟪 D. Ketones
1️⃣1️⃣ Product when HBr is added to ethyne
🟦 A. 1,1-Dibromoethane
🟩 B. 1,2-Dibromoethane
🟧 C. 1,1,2,2-Tetrabromoethane
🟪 D. Bromoethane
1️⃣2️⃣ Ozonide on heating with Zn-dust gives
🟦 A. Aldehyde
🟩 B. Alcohol
🟧 C. Alkene
🟪 D. Ether
1️⃣3️⃣ Reagent to distinguish ethene & ethyne
🟥 A. Ammoniacal AgNO₃
🟦 B. Acidified KMnO₄
🟧 C. Bromine water
🟩 D. Alkaline KMnO₄
1️⃣4️⃣ C–C sigma bond in ethane forms by overlap of
🟦 A. sp³–sp³
🟩 B. sp²–sp²
🟧 C. sp–sp
🟪 D. s–sp³
1️⃣5️⃣ Cycloalkanes have lower boiling points because of
🟥 A. Ring strain
🟩 B. sp³ hybridization
🟧 C. Tetrahedral structure
🟦 D. Strong IMF
1️⃣6️⃣ HBr addition to propyne gives
🟦 A. 1,1-Dibromopropane
🟩 B. Bromopropane
🟧 C. 1,1,2,2-Tetrabromopropane
🟪 D. 2,2-Dibromopropane
1️⃣7️⃣ C–C σ-bond in ethene is formed by
🟦 A. sp³–sp³
🟩 B. sp²–sp²
🟧 C. sp–sp
🟪 D. s–sp³
1️⃣8️⃣ A saturated hydrocarbon with formula C₃H₆ must be
🟦 A. Propyne
🟧 B. Cyclopropane
🟩 C. Propane
🟪 D. Propene
1️⃣9️⃣ Welding gas is
🟦 A. Ethylene
🟥 B. Acetylene
🟩 C. Ethane
🟧 D. Methane
2️⃣0️⃣ Smallest and simplest cycloalkane
🟥 A. Cyclobutane
🟧 B. Cyclopropane
🟩 C. Cycloethane
🟦 D. Cyclopentane
✅ Answer Key
1️⃣ C
2️⃣ C
3️⃣ C
4️⃣ A
5️⃣ C
6️⃣ C
7️⃣ C
8️⃣ D
9️⃣ B
🔟 A
1️⃣1️⃣ A
1️⃣2️⃣ A
1️⃣3️⃣ A
1️⃣4️⃣ A
1️⃣5️⃣ A
1️⃣6️⃣ D
1️⃣7️⃣ B
1️⃣8️⃣ B
1️⃣9️⃣ B
2️⃣0️⃣ B
🌱💥 Top-Scoring Hydrocarbons MCQs | Past Paper Style, Exam-Level Practice
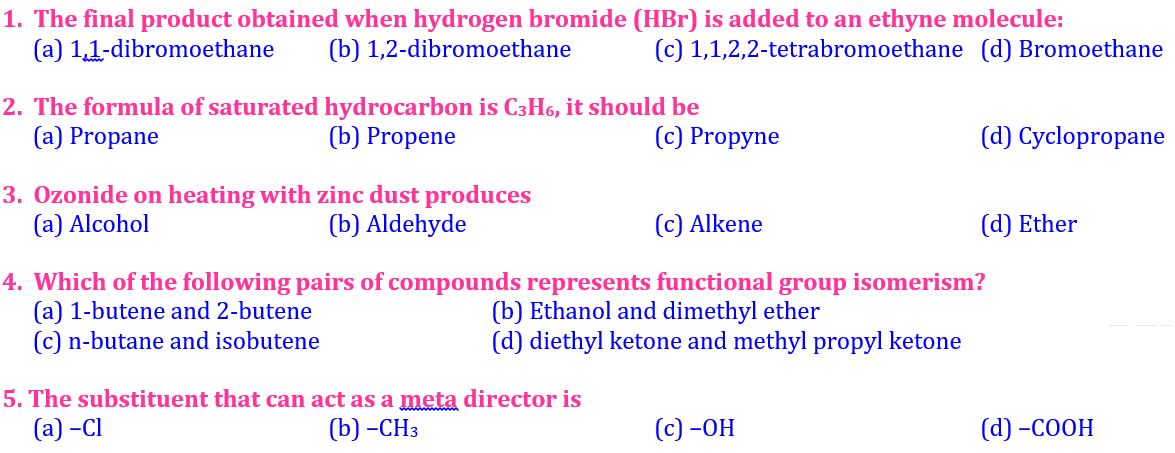
1️⃣ The substituent that can act as a meta director is:
🟨 A –Cl
🟩 B –CH₃
🟦 C –OH
🟪 D –COOH
2️⃣ Acylation of benzene in the presence of AlCl₃ gives:
🟨 A Toluene
🟩 B Acetophenone
🟦 C Phenol
🟪 D Xylene
3️⃣ Benzene adds 3 molecules of chlorine gas in presence of sunlight gives:
🟨 A Benzene hexachloride
🟩 B Hexachloro cyclohexane
🟦 C Lindane
🟪 D All of them
4️⃣ Which of the following groups do NOT direct the 2nd substituent to the meta position?
🟨 A Nitro group
🟩 B Sulphonic acid group
🟦 C Halide group
🟪 D Carboxylic group
5️⃣ The C-C bond length in benzene is:
🟨 A 1.54Å
🟩 B 1.39 Å
🟦 C 1.20 Å
🟪 D 1.34 Å
6️⃣ Shape of benzene molecule is:
🟨 A Linear
🟩 B Pyramidal
🟦 C Planar trigonal
🟪 D Planar hexagonal
7️⃣ In which case the C-C bond length is same:
🟨 A Benzene
🟩 B Propyne
🟦 C 1-butene
🟪 D 2-butene
8️⃣ Which type of overlapping forms the C-C bond in benzene?
🟨 A sp³-sp³
🟩 B sp²-sp²
🟦 C sp-sp
🟪 D sp³-s
9️⃣ Which one of the following is polycyclic compound?
🟨 A Styrene
🟩 B Cumene
🟦 C Naphthalene
🟪 D Xylene
1️⃣0️⃣ Anhydrous AlCl₃ is used in the Friedal-Craft’s reaction because it is:
🟨 A Soluble in ether
🟩 B Insoluble in benzene
🟦 C Electron deficient
🟪 D Electron rich
1️⃣1️⃣ The compound that is nitrated with difficulty is:
🟨 A Phenol
🟩 B Benzene
🟦 C Toluene
🟪 D Nitrobenzene
1️⃣2️⃣ Which of the following compounds reacts slower than benzene in electrophilic substitution?
🟨 A C₆H₅-CHO
🟩 B C₆H₅-NH₂
🟦 C C₆H₅-CH₃
🟪 D C₆H₅-OH
1️⃣3️⃣ The ratio of sigma and pi bonds in benzene is:
🟨 A 2
🟩 B 6
🟦 C 8
🟪 D 4
1️⃣4️⃣ Benzene contains:
🟨 A 4π electrons
🟩 B 3π electrons
🟦 C 8π electrons
🟪 D 6π electrons
1️⃣5️⃣ Six carbon atoms of benzene are of:
🟨 A Two types
🟩 B Three types
🟦 C Six types
🟪 D One type
1️⃣6️⃣ During nitration of benzene the active nitrating agent is:
🟨 A HNO₃
🟩 B NO₂⁻
🟦 C NO₃⁻
🟪 D NO₂⁺
1️⃣7️⃣ Amongst the following the strongest ortho-para directing group is:
🟨 A –Cl
🟩 B –OH
🟦 C –Br
🟪 D –C₆H₅
🟨 A –Cl
🟩 B –OH
🟦 C –Br
🟪 D –C₆H₅
1️⃣9️⃣ In benzene sulphonic acid the sulphonic group is attached with benzene ring through:
🟨 A OH
🟩 B Sulphur
🟦 C Hydrogen
🟪 D Oxygen
2️⃣0️⃣ Aniline is a derivative of benzene which contains:
🟨 A Amide group
🟩 B Amino group
🟦 C Imino group
🟪 D Nitro group
2️⃣1️⃣ Acetophenone is a/an:
🟨 A Ether
🟩 B Ketone
🟦 C Ester
🟪 D Aldehyde
2️⃣2️⃣ During sulphonation of benzene H₂SO₄ generates the electrophile:
🟨 A HSO₄⁻
🟩 B SO₂
🟦 C SO₃
🟪 D H⁺
2️⃣3️⃣ Which of the following reactions would benzene is expected to undergo?
🟨 A Addition
🟩 B Elimination
🟦 C Substitution
🟪 D Cracking
2️⃣4️⃣ –SO₂OH group is called:
🟨 A Carbonyl group
🟩 B Sulphuryl group
🟦 C Sulphonic group
🟪 D Phenyl group
2️⃣5️⃣ Xylene has ________ isomers:
🟨 A 2
🟩 B 1
🟦 C 3
🟪 D 4
2️⃣6️⃣ Oxidation of Toluene by alkaline KMnO₄ or acidified K₂Cr₂O₇ gives:
🟨 A Benzoic acid
🟩 B Benz.dioic acid
🟦 C Tartaric acid
🟪 D Citric acid
2️⃣7️⃣ Halogenation of benzene in strong ultraviolet light gives:
🟨 A Addition product
🟩 B Substituted product
🟦 C Oxidized product
🟪 D All of them
✅ Answer Key
🟦1️⃣ – A
🟦2️⃣ – B
🟦3️⃣ – D
🟦4️⃣ – C
🟦5️⃣ – B
🟦6️⃣ – D
🟦7️⃣ – A
🟦8️⃣ – B
🟦9️⃣ – C
1️⃣0️⃣ – C
1️⃣1️⃣ – B
1️⃣2️⃣ – A
1️⃣3️⃣ – B
1️⃣4️⃣ – D
1️⃣5️⃣ – D
1️⃣6️⃣ – D
1️⃣7️⃣ – B
1️⃣8️⃣ – B
1️⃣9️⃣ – B
2️⃣0️⃣ – B
2️⃣1️⃣ – B
2️⃣2️⃣ – C
2️⃣3️⃣ – C
2️⃣4️⃣ – C
2️⃣5️⃣ – C
2️⃣6️⃣ – A
2️⃣7️⃣ – B
🟦2️⃣ – B
🟦3️⃣ – D
🟦4️⃣ – C
🟦5️⃣ – B
🟦6️⃣ – D
🟦7️⃣ – A
🟦8️⃣ – B
🟦9️⃣ – C
1️⃣0️⃣ – C
1️⃣1️⃣ – B
1️⃣2️⃣ – A
1️⃣3️⃣ – B
1️⃣4️⃣ – D
1️⃣5️⃣ – D
1️⃣6️⃣ – D
1️⃣7️⃣ – B
1️⃣8️⃣ – B
1️⃣9️⃣ – B
2️⃣0️⃣ – B
2️⃣1️⃣ – B
2️⃣2️⃣ – C
2️⃣3️⃣ – C
2️⃣4️⃣ – C
2️⃣5️⃣ – C
2️⃣6️⃣ – A
2️⃣7️⃣ – B
🧪⚡ Ultimate Hydrocarbons MCQs Bank | Test Your Concepts Like a Pro
2️⃣1️⃣ The final product obtained when hydrogen bromide (HBr) is added to an ethyne (C₂H₂) molecule:
🟨 A 1,1-dibromoethane
🟩 B 1,2-dibromoethane
🟦 C 1,1,2,2-tetrabromoethane
🟪 D Bromoethane
2️⃣2️⃣ Ozonide on heating with zinc dust produces:
🟨 A Aldehyde
🟩 B Alcohol
🟦 C Alkene
🟪 D Ether
2️⃣3️⃣ Select the suitable chemical to distinguish between ethene (C₂H₄) and ethyne (C₂H₂):
🟨 A Ammonical AgNO₃
🟩 B Acidified KMnO₄
🟦 C Bromine water
🟪 D Alkaline KMnO₄
2️⃣4️⃣ The C–C σ-bond in ethane (C₂H₆) is formed by the linear overlap of:
🟨 A sp³–sp³
🟩 B sp²–sp²
🟦 C sp–sp
🟪 D s–sp³
2️⃣5️⃣ The boiling point of cycloalkanes is lower than that of straight chain alkanes of comparable molar mass due to:
🟨 A Ring strain
🟩 B sp³ hybridization
🟦 C tetrahedral structure
🟪 D strong IMF
2️⃣6️⃣ Which reagent is used for the decarboxylation of sodium alkanoate?
🟨 A Tollen’s reagent
🟩 B Soda lime
🟦 C Soda ash
🟪 D None of them
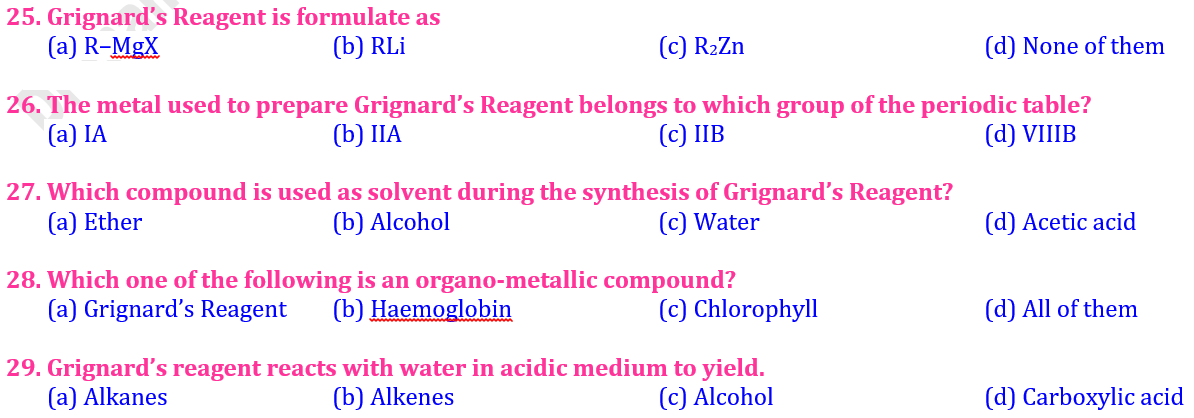
2️⃣7️⃣ The metal used to prepare Grignard’s Reagent belongs to which group of the periodic table?
🟨 A IA
🟩 B IIA
🟦 C IIB
🟪 D VIIIB
2️⃣8️⃣ Which one of the following is an organo-metallic compound?
🟨 A Grignard’s Reagent
🟩 B Haemoglobin
🟦 C Chlorophyll
🟪 D All of them
2️⃣9️⃣ When a neutrally charged molecule is subjected to homolytic fission, ……… are obtained as the product:
🟨 A One free radical
🟩 B Two free radicals
🟦 C Two ions
🟪 D None of them
3️⃣0️⃣ The Wolff–Kishner reduction reduces carbonyl compounds (R₂C=O) to:
🟨 A Hydrocarbons
🟩 B Alcohols
🟦 C Acids
🟪 D Ketones
3️⃣1️⃣ The final product obtained when hydrogen bromide (HBr) is added to a propyne (C₃H₄) molecule:
🟨 A 1,1-dibromopropane
🟩 B 2,2-dibromopropane
🟦 C 1,1,2,2-tetrabromopropane
🟪 D Bromopropane
3️⃣2️⃣ The C–C σ-bond in ethene (C₂H₄) is formed by the linear overlap of:
🟨 A sp³–sp³
🟩 B sp²–sp²
🟦 C sp–sp
🟪 D s–sp³
3️⃣3️⃣ The formula of saturated hydrocarbon is C₃H₆, it should be:
🟨 A Propyne
🟩 B Cyclopropane
🟦 C Propane
🟪 D Propene
3️⃣4️⃣ Welding gas among the following is:
🟨 A Ethylene (C₂H₄)
🟩 B Acetylene (C₂H₂)
🟦 C Ethane (C₂H₆)
🟪 D Methane (CH₄)
3️⃣5️⃣ Which is the smallest and simplest cycloalkane?
🟨 A Cyclobutane (C₄H₈)
🟩 B Cyclopropane (C₃H₆)
🟦 C Cycloethane (C₂H₄)
🟪 D Cyclopentane (C₅H₁₀)
3️⃣6️⃣ The carbon atoms in cyclopropane are sp³-hybridized arranged in a ……… geometry:
🟨 A Linear
🟩 B Tetrahedral
🟦 C Trigonal
🟪 D Angular
3️⃣7️⃣ Grignard’s Reagent is chemically called:
🟨 A Tetraethyl lead
🟩 B Methylcobalamine
🟦 C Alkyl magnesium halide (R–Mg–X)
🟪 D Dimethyl zinc
3️⃣8️⃣ Which compound is used as solvent during the synthesis of Grignard’s Reagent?
🟨 A Alcohol
🟩 B Ether (C₂H₅)₂O
🟦 C Water (H₂O)
🟪 D Acetic acid (CH₃COOH)
3️⃣9️⃣ The intermediate formed during Wolff–Kishner reaction is:
🟨 A Hydrazone
🟩 B Hydrazine (N₂H₄)
🟦 C Oxime
🟪 D Imine
4️⃣0️⃣ Which of the following compounds reacts most readily with Br₂(g)?
🟨 A C₂H₂
🟩 B C₃H₆
🟦 C C₂H₄
🟪 D C₄H₁₀
✅ Answers
2️⃣1️⃣ – A
2️⃣2️⃣ – A
2️⃣3️⃣ – C
2️⃣4️⃣ – A
2️⃣5️⃣ – A
2️⃣6️⃣ – B
2️⃣7️⃣ – A
2️⃣8️⃣ – D
2️⃣9️⃣ – B
3️⃣0️⃣ – A
3️⃣1️⃣ – A
3️⃣2️⃣ – B
3️⃣3️⃣ – B
3️⃣4️⃣ – B
3️⃣5️⃣ – B
3️⃣6️⃣ – C
3️⃣7️⃣ – C
3️⃣8️⃣ – B
3️⃣9️⃣ – A
4️⃣0️⃣ – C
Tags
2nd Year
Class 12 Chemistry
Exam Preparation
Hydrocarbons
MCQs
MDCAT ECAT chemistry
Model Test Questions
Practice Questions
XII Chemistry
XII Chemistry Model Test Questions Chapter # 5