Test Questions on
Chapter # 6, Introduction to Organic Chemistry Test # 1
Q1. Define the following
with examples:
homologous series, functional group, catenation, Polymerization (polymers),
isomerism (isomers), metamersim, knocking, octane number, reforming,
knock-inhibitor, carbonization, heterocylics.
Q2. Write down differences between following with examples and give one chemical tests to identify them:
(i) Aliphatic and aromatic compounds (Soot Test)
(ii) Saturated and unsaturated compounds (BUT)
Q3. Write down Condensed general formulae of following
homologous series:
Alkenes (CnH2n),
Aldehydes (CnH2nO),
Carboxylic acids (CnH2nO2),
Alkynes (CnH2n-2),
Esters (CnH2nO2),
Ketones (CnH2nO),
cylcoalkanes (CnH2n),
cycloalkenes (CnH2n-2).
Q4. Name the alkanes, alkenes and alkynes having the following formula
(i) C2H4 (ethene)
(ii) C3H4 (propyne)
(iii) C2H6 (ethane)
(iv) C6H12 (hexene or cylcohexane)
(v) C5H8 (pentyne)
(vi) C8H16 (Octene)
(vii) C7H12 (Heptane)
(viii) C6H10 (Hexyne)
Q5. Identify the series with following general
formulae:
Q6.
Q6. What are alkyl groups? Give its general formula and type formula. Describe name and structural formulae of butyl and pentyl radical.
Q7. What
is meant by homologous series? Describe 4 general features of homologous series
Q8. Write
down short note on any 5 carbon-containing and 5 oxygen-containing functional
groups.
Q9.Write down names and draw structural
formulae three positional isomers of cresol.
Q10. Write down names and draw structural
formulae three positional isomers of xylenes
Q11. What
is the functional group of ethers? Give their general formula. Write down their
classification with examples.
Q12. What
is the functional group of ketones? Give their general formula. Write down
their classification with examples.
Q13.Write
general formula for aldehydes and carboxylic acids. Name and give formulae of
first six members of aldehydes and carboxylic acids.
Q14. Identify the functional groups in the following compounds:
(a) CH3CHO (Aldehydic group)
(b) CH3CH2CH2OH (primary alcoholic group
(c) CH3COCH3 (ketonic carbonyl group)
(d) CH3COOH (carboxylic group)
(e) CH2=CHCH3 (double bond)
(f) CH3CONH2 (Amide group)
Q15. Write down formulae and class of following functional groups:
Epoxide group, Carbonyl group, Olefinic double bond, Ether linkage, Sulphonic acid group, Nitrile group
Q16.Encircle the
functional groups in the following compounds. Also give the names of the
functional groups?
Q17. Give structural formulae of following compounds:
(1) Picric acid
(2) Pyrogallol
(3) Catechol
(4) Resorcinol
(5) b-naphthol
(6) Hydroquinone
(7) Terephthalic acid
(8) trifluoro acetic acid
(9) diphenyl amine
(10) α-naphthol
(11) isopropyl methanoate
(12) iso-butyric acid
(13) diethyl acetylene
(14) divinyl acetylene
(15) 1,4-hexadiene
(16) tribromoethanal
(17) 4, 5-dimethyl 3-heptanone
(18) Methyl isopropyl ether
(19) Ethyl ethanoate
(20) 3-ethoxyhexane
(21) Ethyl ter-butyl ether
(22) Glyoxal
(23) isobutyraldehyde
(24) p-cresol
(25) Valeric acid
(26) diisopropyl ether
(27) Neopentyl alcohol
(28) Pyrogallol
(29) Caproic acid
(30) Phenylhydrazine
Answers of Test Questions on Chapter # 6, Introduction to Organic Chemistry Test # 1
Q1. Define the
following with examples:
Alkyl group, homologous
series, functional group, catenation, Polymerization (polymers), isomerism
(isomers), metamersim, knocking, octane number, reforming, knock-inhibitor,
carbonization, heterocylics.
Answer
Catenation (a unique
property of carbon)
The property
of carbon atoms to bond or link itself to other carbon atoms forming long
chains, branched chains, rings or compounds with chains and rings together is
called Catenation.
Alkyl Radicals
Alkyl groups are the basic structural
unit of all aliphatic organic compounds. The residual hydrocarbon group or
radical left after the removal of a hydrogen atom from a saturated hydrocarbon
alkane is called an alkyl group or radical.
Homologous Series
The members of the same class of organic
compounds arranged in order of ascending molecular masses having same
functional group whose successive members differ from each other by an integral
number of methylene groups (–CH2–) in their molecular formulae (or
by molecular mass of 14) are said to form a Homologous Series.
Functional Group
An atom or group of atoms which is
present within the organic molecule and is responsible for its chemical
behaviour and characteristic properties is called Functional Group.
Polymerization
(polymers)
It is a
chemical reaction in which a large number of smaller molecules called monomers
react together to form a larger molecule called polymer that contains repeating
structural units. The macromolecules formed
may have a linear or branched structure with complex three-dimensional network.
isomerism (isomers)
Isomerism is
the existence of different compounds exhibiting different physical or chemical
properties or both having same molecular formula i.e. The phenomenon of
existence of two or more compounds possessing the same molecular formula but
different properties is known as isomerism. Such compounds are called as
isomers.
metamersim
Isomerism resulting from unequal
distribution of carbon atoms (alkyl or aryl groups) on either side of the
polyvalent functional group is called metamerism.
Different compounds which have same
molecular formula having same functional group in which polyvalent atom of the
same functional group joins different combinations of alkyl or aryl radicals
are called metamers.
knocking
A sharp metallic sound or tapping
noise produced in the internal combustion engine due to pre-ignition or
premature combustion of the fuel in the cylinder prior to sparking is called
Knocking or Pinking.
octane number
it is the capacity of a sample of gasoline
to resist knocking expressed as a number equal to the percentage of isooctane
in an isooctane-heptane mixture that has the same knocking characteristics.
reforming
The process in which the atoms are
rearranged in straight chain alkanes (obtained from gasoline fractions) to
produce branched chain alkanes or aromatic hydrocarbons is known as Reforming
or Isomerisation. It is the conversion of straight hydrocarbon into branched
chain hydrocarbons in order to increase the octane number of a fuel to avoid
knocking.
knock-inhibitor
The knock inhibitors or anti-knocking
agent (acting as Catalysts) like tetraethyl Lead, TEL [(C2H5)4Pb)
greatly reduce knocking by enhancing octane number of fuel.
carbonization
Thermal decomposition of a highly
carbonaceous material such as coal in the absence of air resulting in
decomposition to solids, liquids and gases is called Carbonization or
Destructive Distillation.
heterocylics
The cyclic compounds having one or more hetero atoms (like nitrogen,
sulphur or oxygen) along with carbon in the ring are called Heterocyclic or
Non-Carbocyclic Compounds e.g. Pyrole/Ozole, Furan/Oxole, Thiophene/thiole, Pyridine/Azine
Q2.Write down difference between following with examples and give two chemical tests to identify them:
(i) Aliphatic and aromatic compounds (Soot
Test)
(ii) Saturated and unsaturated compounds
(BUT)
Answer
Difference between
Aliphatic and Aromatic Compounds
Difference between
Aliphatic and Aromatic Compounds
Difference between Saturated and Unsaturated Hydrocarbons
Q3. Write down Condensed general formulae of following
homologous series:
Answer
Alkenes ………………….. CnH2n)
Aldehydes………………. (CnH2nO)
Carboxylic acids …….. (CnH2nO2)
Alkynes ………………… (CnH2n-2)
Esters ………………….. (CnH2nO2)
Ketones ……………….. (CnH2nO)
Cylcoalkanes………… (CnH2n)
Cycloalkenes………… (CnH2n-2).
Q4. Name
the alkanes, alkenes and alkynes having the following formula
Answer
(i) C2H4 (ethene)
(ii) C3H4
(propyne)
(iii) C2H6
(ethane)
(iv) C6H12
(hexene or cylcohexane)
(v) C5H8
(pentyne)
(vi) C8H16
(Octene)
(vii) C7H12
(Heptane)
(viii) C6H10 (Hexyne)
Q5. Identify the series with
following general formulae:
Answer
CnH2n [n = 2 to
infinity]……………….... Alkene
CnH2nO2
[n = 1
to infinity]………………… Acids
CnH2n+2O
[n = 2 to
infinity]…………….. ... Alkane
CnH2nO [n = 3 to infinity]…………………. Ketones or oxirane
CnH2n+1CONH2
[n = 1 to infinity]………………….
Acid amide
CnH2nO2 [n
= 2 to infinity]………………… Ester
CnH2n+1CONH2 [n
= 1 to infinity]………………… Acid amide
CnH2n+2O [n
= 1 to infinity]…………………. Alcohols
CnH2n
[n
= 2 to infinity]…………………. Alkene
CnH2nO2 [n
= 1 to infinity]………………… Aldehydes
Q6. What
are alkyl groups? Give its general formula and type formula. Describe name and
structural formulae of butyl and pentyl radical.
Answer
Definition of Alkyl
Radicals
Alkyl groups are the basic structural unit of all aliphatic organic
compounds.
The residual hydrocarbon group or radical left after the removal of
a hydrogen atom from a saturated hydrocarbon alkane is called an alkyl group or
radical. Stated differently, the radicals
obtained from alkanes by the removal of one hydrogen atom are called alkyl
group or radical. The smallest alkyl group is CH3– called
methyl.
Alkyl radicals are derivatives
of alkanes. Alkanes are quite often represented as R-H and
here R stands for alkyl group. Alkyl groups are generally represented by ‘R–‘.
Butyl Group; (C4H9–); It may exist in 4 isomeric forms:
It is derived from butane (C4H10)
having formula C4H9–.It
may exist in 4 isomeric forms.
(v) Pentyl Group or Amyl group (C5H11–); It may exist in 8 isomeric forms:
Pentyl group (C5H11–) has the following 8
isomers, 4 are primary, 3 are secondary and 1 is tertiary:
Q7. What is meant by
homologous series? Describe 4 general features of homologous series
Answer
Definition
“The members of the same class of organic compounds arranged in
order of ascending molecular masses having same functional group whose
successive members differ from each other by an integral number of methylene
groups (–CH2–) in their molecular formulae (or by molecular mass of
14) are said to form a Homologous Series.”
For example; In the first ten members of alkanes (C1 – C10),
each member differs from its neighbour by –CH2– group. There is a
gradual change in their physical properties with increasing molecular weight
but chemical properties like combustion, halogenation are same.
e.g.
General characteristics of homologous series
1. Identical
structures and common difference in composition
All members of a homologous series have identical structures.
Successive members of a series always differ in composition by an integral
number of methylene groups (–CH2–). The difference between the
molecular weights of two successive members of a series will be of 14 amu (u).
The name of each member of a series begins either with a common prefix or
suffix.
2. General Molecular
Formula
All members of a homologous series can be expressed by a general
molecular formula. For example:
3. General Method of
Preparation
All members of a homologous series can be prepared by a common
general method of preparation
e.g.
all members
of alkanes can be prepared by reduction of alkyl halide by nascent hydrogen
[H]:
4. Identical Chemical
Properties
The elements and functional group present in compound of a
homologous series are same. Homologues show almost identical chemical
properties due to the presence of identical functional group.
e.g.
all alkanes
are unreactive under ordinary conditions. They undergo combustion and
substitution reaction with halogens.
Q8. Write down short note on
any 5 carbon-containing and 5 oxygen-containing functional groups.
Answer
1. Ketones; (>C=O)
It is the series of organic compounds in which carbonyl group is
directly linked to two carbon atoms of alkyl radicals. e.g.
(i) H3C–CO–CH3 (Acetone or Propanone)
(ii) C2H5–CO–CH3 (2-butanone)
2. Aldehydes; (–CHO)
It is the series of organic compounds in which carbonyl group is directly linked to at least one hydrogen atom (except formaldehyde).
e.g.
O
||
3. Carboxyl Group ( –C–OH or
–COOH)
It is the combination of a carbonyl group and a hydroxyl group i.e. –CO–OH or –COOH. Carboxyl group is the functional group of carboxylic acids.
e.g.
Following are the derivatives functional groups of carboxyl groups:
(a) Acid
Halides (–CO–X)
(b) Acid
Amides (–CONH2)
(c) Ester
Group (–COO–R)
(d) Acid anhydride
(–CO– O – CO –)
(e) Nitrile group (–CN or –CºN)
(f) Isonitrile group
(–NC or – N+ º C-)
(a). Acid Halides/Halocarbonyl/oylhalide
(–CO–X)
The replacement of hydroxyl group from a carboxyl group by a
halogen atom gives an acid halide group. It is the functional group of acid
halides/Alkanoyl Chloride.
e.g.
(i) H–CO–Cl …………… Formyl chloride (Methanoyl Chloride)
(ii) H3C–CO–Cl
……… Acetyl chloride (Ethanoyl Chloride)
(iii) C2H5–CO–Cl
…… Propionyl chloride (Propanoyl Chloride)
(b). Acid Amides or Amide (–CONH2)
The replacement of hydroxyl group from a carboxyl group by an amino
group gives an amide group. It is the functional group of alkanamide family.
e.g.
(i) H–CO–NH2
………….. Formamide/Methanamide
(ii) CH3–CO–NH2
………. Acetamide/Ethanamide
(i) Primary amides ; (–CONH2) (R–CONH2) ;
e.g. CH3–CONH2 , CH3–CH2–CONH2
(ii) Secondary amines ; (>CONH) (R–CONH–R) ;
e.g. (CH3)2.CONH
(iii) Tertiary amine ; (àCON) (R–CONR2) ;
e.g. CH3–CON.(CH3)2
Like amines,
the amides are classified as primary, secondary and tertiary based on the
number of carbons connected to the nitrogen.
(c). Ester Group (–COO–R)
The replacement of hydrogen of hydroxyl group from a carboxyl group
by an alkyl group (–R) gives an ester group.
It is the functional group of esters.
e.g.
(i) H–COO–CH3 …………. Methyl formate (methyl methanoate)
(ii) CH3–COO–CH3 ………….. Methyl acetate (methyl ethanoate)
(iii) CH3–COO–C2H5 ………….. Ethyl acetate (ethyl
ethanoate)
(d). Carboxylic acid anhydride/Acid anhydride group;
(–CO– O – CO –)
It is the functional group of acid anhydride family.
e.g.
(i) H–CO–O–CO –H ……………. Formic Anhydride
(ii) CH3–CO–O–CO–CH3 …………… Acetic Anhydride
(e). Cyanide or
Nitrile group (–CN or –CºN)
It is the functional group of alkanenitrile family.
e.g.
(i) CH3–CN
……………. ethanenitrile
(ii) C2H5–CN
…………… propanenitrile
(f). Isocyanide or
Isonitrile or Carbylamine (–NC or – N+ º C-)
It is the functional group of alkaneisonitrile family.
e.g.
(i) CH3–NC ……………..
ethaneisonitrile
(ii) C2H5–NC
…………… propaneisonitrile
Q9. Write down names and
draw structural formulae three positional isomers of cresol.
Answer
Cresol (methylphenol) has three
positional isomers which show different physical properties.
Q10. Write down names and draw
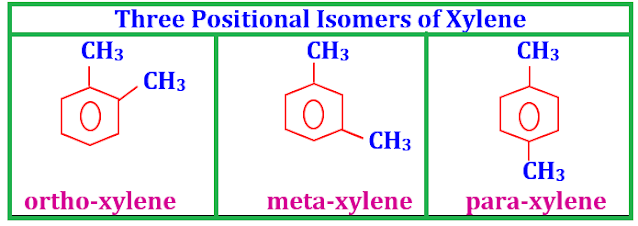
structural formulae three positional isomers of xylenes.
Answer
Di-substituted benzenes (DSB) have
three positional isomers namely ortho, meta and para e.g. xylene (a DSB) has
three positional isomers.
Q11. What
is the functional group of ethers? Give their general formula. Write down their
classification with examples.
Answer
Ether Linkage
The linkage of two carbon atoms through an oxygen atom is called an Ether linkage (C – O – C). It is the functional group of ether family.
e.g.
(i) CH3–O–CH3
…………………. (dimethyl ether)
(ii) C2H5–O–CH3
……………….. (ethyl methyl ether)
(iii) C2H5–O–C2H5
……………… (diethyl ether)
Q12. What
is the functional group of ketones? Give their general formula. Write down
their classification with examples.
Answer
(a) Ketones; (>C=O)
It is the series of organic compounds in which carbonyl group is
directly linked to two carbon atoms of alkyl radicals. e.g.
(i) H3C–CO–CH3
…………. (Acetone or Propanone)
(ii) C2H5–CO–CH3
…..……. (2-butanone)
Q13.Write
general formula for aldehydes and carboxylic acids. Name and give formulae of
first six members of aldehydes and carboxylic acids.
Answer
General formula of Aldehydes
CnH2n+1–
CHO/ CnH2nO (isomeric with ketone/oxirane/alkenal etc.)
First Six Members of Aldehydes
Formaldehyde…………………….. (H – CHO)
Acetaldehyde……………………… (H3C– CHO)
Propionaldehyde…………………(C2H5– CHO)
Butyraldehyde……………………..(C3H7– CHO)
Valeraldehyde……………………… (C4H9– CHO)
Caproaldehyde……………………..(C5H11– CHO)
General formula of Carboxylic Acids
CnH2n+1–
–COOH / CnH2nO2 (isomeric with ester)
First Six Members of Carboxylic Acids
Formic acid (H
– COOH)
Acetic acid (H3C–
COOH)
Propionic acid (C2H5–
COOH)
Butyric acid (C3H7–
COOH)
Valeric acid (C4H9–
COOH)
Caproic acid (C5H11–
COOH)
Q16. Encircle the functional groups in
the following compounds. Also give the names of the functional groups?
Answer
Q17. Give structural formulae of following compounds: