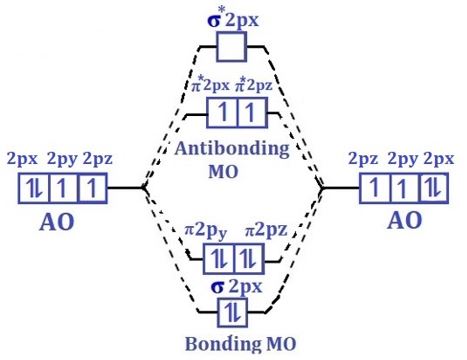
Molecular orbital Diagram for O2 molecule
The electronic configuration of oxygen (Z=8) is 1s2, 2s2,
2p4 (1s↿⇂, 2s↿⇂, 2px↿⇂ 2py↿
2pz↿) Thus there are five atomic
orbitals with 8 electrons in each oxygen atom. The two participating oxygen atoms
contribute a total 16 valence electrons. These five atomic orbitals of both
oxygen atoms combine to form ten molecular orbitals as shown in
molecular orbital energy diagram.
Two p-atomic orbitals (one from
each oxygen) atom combine to form two molecular orbitals, the bonding molecular
orbital σ2px and antibonding molecular orbital σ*2px. The
other four p-atomic orbitals (two from each oxygen) atom combines to give four
molecular orbitals, two bonding molecular orbitals i.e. π2py and π2pz,
while two antibonding molecular orbitals i.e. π*2py and π*2pz.
The electron filling in these molecular orbitals follows Aufbau, Pauli
exclusion principle and Hund’s rule.
Out of eight electrons, six go
to bonding molecular orbitals and two to the antibonding molecular orbitals. As
electrons are also present in antibonding molecular orbitals, so weak bonds
will be formed.
Bond Order
Bond order of O2 molecules
is determined as
Nb – Na/2 = 10–6 /2 = 2 (Double bond, O=O)
OR
We can calculate bond order by considering electrons in p-orbitals as
Nb – Na/2 = 6–2 /2 = 2
As the bond order in Oxygen is 2 so two
bonds i.e. double bonds formed between two oxygen atoms (O=O).
Reason of Paramagnetic Nature of
O2 molecule
Since there are two unpaired electrons in degenerate anti-bonding
molecular orbital (π*2py and π*2pz), O2
molecule is paramagnetic in nature.
molecular orbital configuration with increasing energy order
𝜎1s↿⇂ < 𝜎*1s↿⇂ < 𝜎2s↿⇂ < 𝜎*2s↿⇂ < < 𝜎
2px↿⇂ < 𝜋2py↿⇂ = 𝜋2pz↿⇂ < 𝜋*2py↿
= 𝜋*2pz↿ < 𝜋*2px
Summary of MO Diagram of O2
Given element – Oxygen
Atomic number of Oxygen = 7
Electronic configuration of O atom = 1s↿⇂,
2s↿⇂, 2px↿⇂ 2py↿ 2pz↿
Total number of electrons in Oxygen molecule = 16 (8 by each O)
Electronic
configuration of O2 =
𝜎1s↿⇂ < 𝜎*1s↿⇂ < 𝜎2s↿⇂ < 𝜎*2s↿⇂ < < 𝜎
2px↿⇂ < 𝜋2py↿⇂ = 𝜋2pz↿⇂ < 𝜋*2py↿
= 𝜋*2pz↿ < 𝜋*2px
Bond order of
O2 molecules = Nb – Na/2 = 10–6 /2 = 2 (Double
bond, O=O)
Paramagnetic nature
= Two unpaired electrons in anti-bonding orbitals, hence O2 is
paramagnetic.
Molecular orbital Diagram for N2 molecule
The electronic configuration of nitrogen (Z=7) is 1s2, 2s2,
2p3 (1s↿⇂, 2s↿⇂, 2px↿ 2py↿
2pz↿) Thus there are five atomic
orbitals with 7 electrons in each nitrogen atom. The two participating nitrogen
atoms contribute a total 14 valence electrons. These five atomic orbitals of
both nitrogen atoms combine to form ten molecular orbitals as shown in
molecular orbital energy diagram.
Two p-atomic orbitals (one from
each nitrogen) atom combine to form two molecular orbitals, the bonding
molecular orbital σ2px and antibonding molecular orbital σ*2px.
The other four p-atomic orbitals (two from each nitrogen) atom combines to give
four molecular orbitals, two bonding molecular orbitals i.e. π2py and
π2pz, while two antibonding molecular orbitals i.e. π*2py
and π*2pz. The electron filling in these molecular orbitals follows
Aufbau, Pauli exclusion principle and Hund’s rule.
Out of seven electrons, five go
to bonding molecular orbitals and two to the antibonding molecular orbitals. All
six electrons of p-orbitals go to bonding molecular orbitals, so strong bonds
will be formed.
Bond order
Bond order of N2 molecules
is determined as
Bond order = Nb – Na/2
= 10 – 4/2 = 3
OR
Bond order = Nb – Na/2
= 6 – 0/2 = 3
As the bond order in nitrogen is 3 so three
bonds i.e. triple bonds formed between two nitrogen atoms (N≡N).
Reason of Diamagnetic Nature of N2
molecule
Since there is no unpaired electron in any of the molecular orbitals of nitrogen
molecule, hence N2 molecule is diamagnetic in nature.
molecular orbital configuration with increasing energy order
𝜎1s
< 𝜎*1s < 𝜎2s < 𝜎*2s < 𝜋2py = 𝜋2pz
< 𝜎 2px < 𝜋*2py = 𝜋*2pz
< 𝜋2px
Summary of MO Diagram of N2
Given element – nitrogen
Atomic number of nitrogen = 7
Electronic configuration of N atom = 1s↿⇂,
2s↿⇂, 2px↿ 2py↿ 2pz↿
Total number of electrons in nitrogen molecule = 14
(7 by each N)
Electronic configuration of N2
=
𝜎1s
< 𝜎*1s < 𝜎2s < 𝜎*2s < 𝜋2py = 𝜋2pz
< 𝜎 2px < 𝜋*2py = 𝜋*2pz
< 𝜋2px
Bond order of N2 molecules =
Nb – Na/2 = 10–4 /2 = 3 (Triple bond, N≡N)
Diamagnetic nature = No unpaired
electrons in any orbitals, hence N2 is diamagnetic.