Numericals on Radius, Energy, Frequency, wavelength and wave number from Textbook
Q1. Find
the radius of 4th orbit of electron in Hydrogen atom (Book question;
page # 24, Example # 2.1)
Solution
Z =
1 (for H atom)
‘n’ = 4 (4th orbit)
‘ao’= 0.529 Å
Q2. Calculate the energy of an electron in L-shell of hydrogen atom. The value of K 2.18 x 10−18 J/atom
(Book
question; page # 25, Example # 2.2)
Q3. Using Bohr model, determine the energy in joule of a photon produced when an electron in hydrogen atom jumps from an orbit n=5 to n=2.
(Book
question; page # 26, Self-Assessment)
Solution
‘n2’ = 5
‘n1’ = 2
Q4. Calculate the wave numbers of photons when electron of a hydrogen atom jumps form 4th orbit to 2nd orbit. value of RH = 1.09678 x 107 m−1.
(Book question; page #
27, Example 2.3)
Solution
‘n2’ = 4
‘n1’ = 2
Z =
1
RH = 1.09678 x 107 m−1
Q5. What is the wave number of a photon produced when an electron falls from n=5 level to n=3 level in hydrogen atom. value of RH = 1.09678 x 107 m−1.
(Book question; page # 28, Self-Assessment)
Solution
‘n2’ = 5
‘n1’ = 3
Z =
1
RH = 1.09678 x 107 m−1
HOT FAVOURITE QUESTION
*Q6*. A photon of wave number 23 x 105 m−1 is emitted when electron undergoes a transition from a higher orbits to n=2. Determine the orbit form which electron falls and also the spectral line appears in this transition of electron (The value of Rydberg constant is 1.09678 x 107 m−1).
(Book question; page # 49,
Assignment)
Solution
RH = 1.09678 x 107 m−1
Z =
1
Numericals on Radius, Energy,Frequency, Wavelength and Wave Number from External Source
Q1. If the radius of first Bohr orbit is ‘x’ then calculate the radius of the third orbit of H atom. Solution
For given part ‘n’ = 1 ao = r1= 0.529 Å= ‘x’ r3 = ? Radius (r3) = (x) x 32/1 Å Þ = (x) x 9/1 Å Þ r3 = 9x Å
The radius of the third orbit of H atom is 9 times
than that of radius of first orbit.
Q2. If the radius of first Bohr orbit is ‘x’ Å then calculate the radius of the 2nd orbit of hydrogen. SolutionZ = 1 (for H atom)
‘n’ = 2 (2nd orbit)
‘ao’= ‘x’ Å
The radius of the second orbit of H atom is 4 times
than that of radius of first orbit.
Q3.Calculate the ratio of radius of second and third orbit of hydrogen atom.
(Bohr’s radius = 0.529 Å)
Solution
Z = 1 (for H atom)
‘n’ = 2 (2nd orbit)
‘n’ = 3 (3rd orbit)
‘ao’= 0.529 Å
Q4. Calculate the ratio of
radius of third and fourth orbit of hydrogen atom. (Bohr’s radius = 0.0529 nm)
Solution
Z = 1 (for H atom)
‘n’ = 3 (3rd orbit)
‘n’ = 4 (4th orbit)
‘ao’= 0.0529 nm
Q5.Calculate the ratio of the radius of Li2+ ion in 3rd
energy level to that of He+ ion 2nd energy level.
Solution
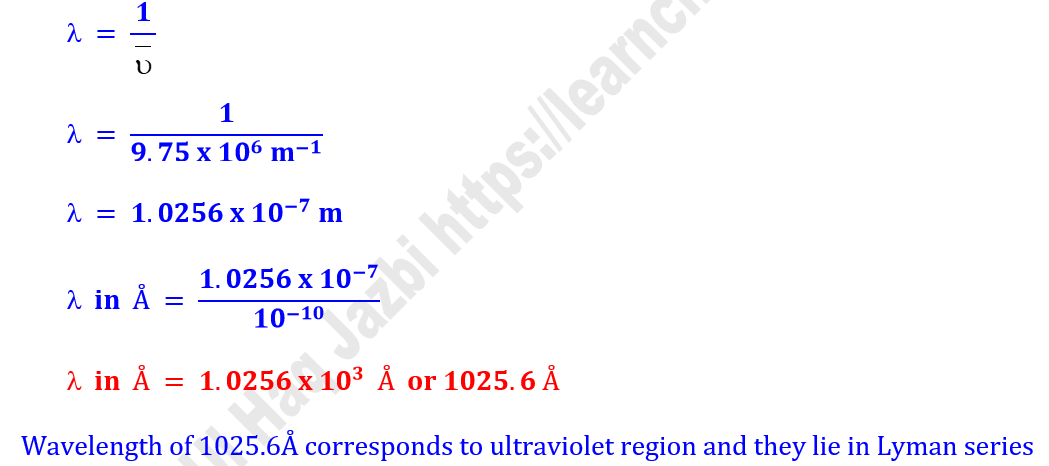
Q6.What is the wavelength and wave number of
radiation that is emitted when a hydrogen atom undergoes a transition from
orbit 3 to orbit 1.
Solution
‘n2’
= 3
‘n1’ = 1
Z = 1
RH
= 1.09678 x 107 m−1
Calculation of Wave Number
Calculation of Wave Length
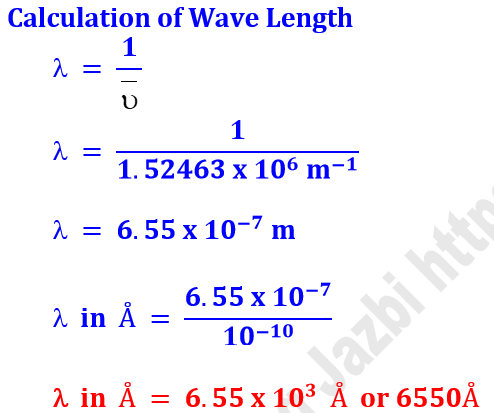
Q7. Calculate the wave number and wavelength of Balmer series of
hydrogen spectrum in which electron jumps from orbit 3 to orbit 2.
Solution
Calculation of Wave Number
n1 = 2
n2 = 3
RH = 1.0968553 x 107 m-1
Z = 1
Q8. Calculate
the energy of the electron in the ground state and first excited state of the
hydrogen atom
Solution
Z for H = 1
n for ground state
= 1
n for first
excited state = ground state +1 = 1+1=2 (corresponds second energy level)
‘K’=
2.18 x 10−18 J/atom or 13.6 eV
Q9. The energy
of electron in the excited state of hydrogen atom is -0.85 eV. calculate
the value of ‘n’ for
in the excited state.
Assignment
Q1. Calculate the energy of 1st, 2nd and 3rd
orbit of hydrogen atom.
(Answers; -13.6 eV, -3.41 eV,
-1.51 eV)
Q2. Calculate the radius of 1st, 2nd and 3rd
orbit of hydrogen atom.
(Answers; 0.529°A, 2.226°A,
4.761°A)
Q3. Calculate the angular momentum of 1st and 2nd
orbit of hydrogen atom.
(1.054 x 10−34 Js,
2.108 x 10−34 J.s)
Q4. Calculate the wave number of the line in Lyman series when
an electron jumps from orbit 3 to orbit 1.
(Answer; 97613.4 cm−1)
Q5. Calculate the wave number of spectral line of hydrogen gas
when an electron jumps from n = 4 to n = 2.
(Answer; 20654.6 cm−1)