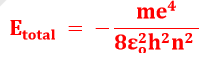XI Chemistry Test Model Questions for
Chapter # 2 (Atomic structure)
Long Questions-Answers
Q10. What are the defects in Rutherford’s atomic model. State the postulates of Bohr’s theory. How did Bohr’s theory explain the formation of the line spectrum of hydrogen atom?
Q11. Write down Defects/ Limitations of Bohr’s theory or Bohr’s atomic model
Q12. Write down 5 spectral lines of series of atomic emission spectrum of hydrogen in tabular form with diagram. Write names and formulae each series with their regions in electromagnetic spectrum.
Q13. Differentiate between Balmer Series and Lyman Series. Explain hydrogen spectrum in terms of Bohr’s theory
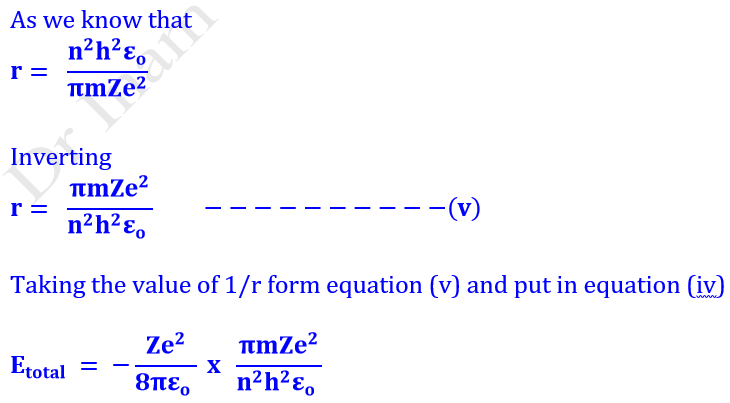
Q14. Derive the formula for the radius and energy of nth orbit of Hydrogen atom.
Q15. Derive the formula for the energy of nth orbit of Hydrogen atom.
Q16. Derive an expression for the frequency of radiation emitted from an electron. Given that
Solutions of Short Questions-Answers Test # 2
Q14. Derive the formula for the radius and energy of nth orbit of Hydrogen atom.
Assumptions for Simple Atom
To derive an expression for radius, consider a hydrogen atom (or hydrogen-like atoms such as He+, Li2+, Be3+, B4+, C5+) with atomic number equal to Z consisting of a single electron with charge –e and mass m revolving around the nucleus of charge +Ze (+e is charge of proton) with a tangential velocity v in the orbit whose radius is r.
Now revolving electron is being acted upon simultaneously by the following two types of forces;
(i) Electrostatic force of Attraction / Centripetal Force
According to Coulomb’s law, the electrostatic force of attraction (Fe) between the nucleus of charge ‘+Ze’ and electron of charge ‘–e’ separated by a distance ‘r’ is given by:
Where ‘K’ is proportionality constant. It is equal to 1/4πεor2
Hence attractive force between nucleus and electron can be written as
(ii) Centrifugal Force
This Coulombic force of (Fc) supplies the centrifugal force to keep the electron in an orbit and is given by:
Equating Fe and Fc
To keep the electron in the same orbit, these two opposite forces must be equal to each other i.e.
Determination of v2 of electron by using Bohr’s Postulate
According to Bohr’s postulate, angular momentum of electron revolving around the nucleus is an integral multiple of h/2p.
Calculation of Radius (r)
Substituting the value v2 from equation (ii) in equation (i)
Where
h = Planck’s constant = 6.625 x 10−34 J.s
me = Mass of electron = 9.11 x 10−31 kg
e = Charge of electron = 1.602 x 10−19 C
𝛆o = Vacuum permittivity constant = 8.84 x 10−12 C2/J.m
Calculation of nth Bohr’s orbit (rn)
Assembling all constants in equation (iii), we get
Where a is known as Bohr’s constant or Bohr radius and its value is 0.529 x 10−8 cm or 0.529 Å or 0.0529nm or 52.9 pm. This is the radius of the first orbit of H. This equation is used for the determination of nth orbit of hydrogen atom and hydrogen like ions like He+, Li2+ etc.
The above equation shows that radius of orbit is directly proportional to the square of the principal quantum numbers (r α n2 i.e. 1, 2, 3, ……..) and inversely proportional to atomic number. As the value of n increases, the radius of the orbit will increase.
Answer
Derivation of Energy of the nth Bohr’s Orbit
Basic of Derivation
The total energy of an electron revolving in any orbit around the nucleus is the sum of kinetic energy and potential energy given by,
Etotal = K.E + PE ……………….(i)
Calculation of K.E
The K.E. of electron with mass m revolving around the nucleus with velocity v is given by the following expression;
Now the centrifugal and centripetal forces upon the revolving electron are given as:
At uniform circular equilibrium motion, these two opposite forces must be equal to each other i.e.
Calculation of P.E.
P.E is the work done in bringing the electron from infinity to a point at a distance r from nucleus and can be calculate as
P.E =work done = −force x displacement = −Fe x r
Here negative sign indicates that P.E decreases when electron is brought form infinity to a point at a distance r. Here negative sign indicates a net attractive interaction, giving algebraically lower energy at shorter distance.
Calculation of Total Energy
Etotal = K.E + PE
Here K is a factor assembled by various constant present in energy equation. Its value is 2.18 x 10−18 J/atom or 1312.8 kJ/mol
E is always negative. Negative sign shows that the electron is bound to the atom and energy must be spent in order to remove it from the orbit.
All energy states are bound states as the negative sign indicates. When n = 1; this corresponds to electron at the closest possible distance from the nucleus and at its lowest energy and is called ground state energy. All energy states with value of n higher than 1 are termed as excited states. When n = α then E = 0; which means that the system is unbound and the electron is free.
It should be noted that the energy is increasing as the n (orbits) increasing; however the difference of energy between two orbits is decreasing.
Conclusion
If total energy = − x
Then
KE = + x
PE = − 2x
2.12 Expression for ∆E of Electronic Transition between Orbits
Calculation of ∆E
Let E1 be the energy of n1 orbit and E2 is for n2 orbit. To calculate the energy emitted by atom in the form of radiation when an electron jumps from a higher energy state n2 to lower energy orbit n1; let us make use of the postulate of Bohr’s model; according to which, the emitted energy is written as
Emitted energy = ∆E = E2 – E1
But,
This equation is used for determining the emission or absorption of energy when electron jumps from one orbit to another
Expression for Frequency of Electronic Transition between Orbits
Calculation of ∆E
Let E1 be the energy of n1 orbit and E2 is for n2 orbit. To calculate the energy emitted by atom in the form of radiation when an electron jumps from a higher energy state n2 to lower energy orbit n1; let us make use of the postulate of Bohr’s model; according to which, the emitted energy is written as
Emitted energy = ∆E = E2 – E1
But,
To calculate the frequency (u) of emitted radiations (or photons there in); let us make use of the Bohr’s postulate; according to which:
hu = ∆E = E2 – E1
But
To calculate the frequency (u) of emitted radiations (or photons there in); let us make use of the Bohr’s postulate; according to which:
hu = ∆E = E2 – E1
But
Expression for Wave Number and Wave Length of Radiation
Atomic Spectrum
If the atom gains energy the electron passes from a lower energy
level to a higher energy level, energy is absorbed that means a specific wave
length is absorbed. Consequently, a dark line will appear in the spectrum. This
dark line constitutes the absorption spectrum.
If the atom loses energy, the electron passes from higher to a
lower energy level, energy is released and a spectral line of specific
wavelength is emitted. This line constitutes the emission spectrum.
Hydrogen spectrum is an important example of atomic emission spectrum.
Hydrogen spectrum can be produced by the passage of electric current at a very
low pressure through hydrogen gas in a discharge tube emitting a bluish light
which when viewed through a spectrometer (spectroscope) shows several sharp lines called spectral lines. The bright lines recorded on the
photographic plate constitute the atomic spectrum of hydrogen. The wavelengths of these lines lie in the visible,
ultraviolet, infra-red and far infra-red regions.
The optical spectrum or atomic emission spectrum of hydrogen
comprises of several series of spectral lines that can be classified into six
(five) groups called spectral series each series, known after their discoverer
as the Balmer, Paschen, Lyman, Brackett, Pfund and Humphrey series. The
first four series were discovered before Bohr’s atomic model.
General Formula for Calculating
Wave Number of any series
A general expression for wave number of each spectral line of each
series is given the Rydberg equation: