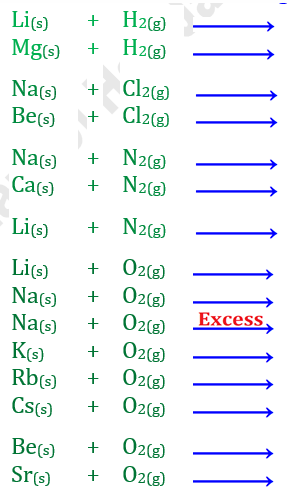Short
Question Answers from Textbook
1. Give reason for the following:
▶ Ionization energy decreases from top
to bottom in s-block elements.
▶ Boiling points of halogens increase
down the group in the periodic table.
▶ Gallium has smaller atomic radii than
aluminium despite being below the aluminium in group IIIA.
▶ Electronegativities of alkali metals
decrease from Li to Cs.
▶ Acidity of hydrogen halides increase
from HF to HI.
▶ Fluorine is the strongest oxidizing
agent.
2. What is
flame test? Mention the colour flame of alkali metals.
3. What is
meant by a diagonal relationship? Mention three pairs of representative
elements that show diagonal relationship.
4. Discus
the group trend of ionization energy in group IIIA and IVA of the periodic
table.
5. Write
down four properties of beryllium that show its unique behaviour in group IIA.
Short
Question Answers from Past Papers
Q1. Write
down three points to show the similarity of diagonal members of group IA and
IIA, IIA and IIIA and IIIA and IVA.
Q2. What
is the basis of flame test? Mention the
colour flame of alkaline earth metals.
Q3. Discus
the group trend of atomic radii in group IIIA of the periodic table
Q4. What is Electrical conductivity? Briefly explain group trend of electrical
conductivity of representative Elements
Q5. Write
down action of oxygen and water on s-block elements with balanced chemical
equation.
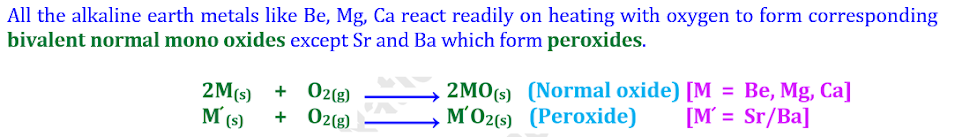
Q6.
Complete and balance the following chemical equations:
Descriptive
Questions
Q1.
Discuss the group trend of atomic radii, melting and boiling points, oxidation
states and electronegativity of representative elements.
Short Question Answers from Past Papers

Answer of Descriptive Questions



Extra Questions