MCQs on
Unit # 1… Mole Concept
Set 1
1. Which
of the following contains the least numbers of molecules?
(a) 1 g of H2
(b) 4 g of O2
(c) 2 g of N2
(d)
3 g of Cl2
Explanation; (Answer; d)
Substance containing least number of moles would
have least number of molecules. Here 3g of Cl2 contains least number
of moles (0.042), so it has least number of molecules.
2. 1 gram molecule of any gas at STP occupies the volume of
(a) 22.4 cm3
(b) 0.0224 dm3
(c) 22400 cm3
(d) 100 cm3
Explanation; (Answer; c)
1 gram molecules constitutes one mole of that
substance. 1 mole of any gas at STP occupies the fixed volume known as molar
gas volume which is equal to 22.4 dm3 or 22400 cm3 or
0.0224 m3.
3. The number of moles of carbon in 200 g of CaCO3 is
(a) 1 mole
(b) 2 mole
(c) 3 mole
(d) 4 mole
Explanation; (Answer; b)
100 g of CaCO3 contains 1 mole of C
200 g of CaCO3 contains 1/100 x 200 = 2
mole of C
4. The volume of semi-mole of a gas at STP is
(a) 11.2 cm3
(b) 11200 cm3
(c) 22.4 ml
(d) 0.0224 m3
Explanation; (Answer; b)
Semi mole means half mole. One mole of any gas STP
occupies 22.4 dm3. Hence half mole of any gas occupies half of 22.4
dm3 i.e. 11.2 dm3. But this is not given in the options.
11.2 dm3 equals to 11200 cm3. So correct option is b.
5. At NTP, 5.6 L of gas weighs 8 grams. The vapour density of a gas is
(a) 32
(b) 40
(c) 16
(d) 8
Explanation; (Answer; c)
We know
that NTP is Normal Temperature and Pressure which is defined as a temperature
of 200C and 1 atmosphere. Vapor density is defined as the
density of a vapor in relation to that of hydrogen. Vapor density can tell us
whether a gas is denser or less dense than air.
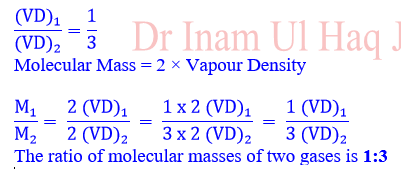
6. The vapour densities of two gases
are in the ratio of 1:3. Their molecular masses are in the ratio of:
(a) 1:3
(b) 1:2
(c) 2:3
(d)
3:1
Explanation; (Answer; a)
7. The number of atoms of Cr and O are 4.8 x 1010 and 9.6 x 1010 respectively. Its empirical formula is
(a) Cr2O3
(b) CrO2
(c) Cr2O4
(d) CrO5
Explanation; (Answer; b)
Alternative method
Molar ratio or atomic ratio of Cr
to O = 4.8 x 1010 : 9.6 x 1010 = 1 : 2
Hence, empirical formula = CrO2
8. A hydrocarbon contains 80% C. The vapour density of compound is 30. The hydrocarbon is
(a) CH3
(b) C2H6
(c) C4H12
(d) C4H8
Explanation; (Answer; a)
Molecular mass = vapour density x
2 = 30 x 2 = 60
Integer (n) = molecular
mass/empirical formula mass = 60/15 = 4
Molecular formula = (EF)n
= (CH3)4 = C4H12
9. Two elements X (atomic weight = 75) and Y (atomic weight = 16) combine to give a compound having 75.8% of X. The empirical formula of the compounds is
(a) Xy
(b) X2Y
(c) X2Y2
(d)
X2Y3
Explanation; (Answer; d)
(a) CHClO
(b) CH2ClO
(c) C2H2ClO
(d) CHCl2O
Explanation; (Answer; a)
11. An unknown gas has a density 1.96 gL−1. Then gas is
(a) CO
(b) NO2
(c) CO2
(d) SO2
Explanation;
Volume of 1 mole of gas at STP = 22.4 L
The molecular mass of CO2 is 12 + 32 = 44 amu.
12. 2.2 g of an unknown gas is present in a container of 1.12 L. Then unknown gas is
(a) CO2
(b) CO
(c) N2O
(d) Both a and c
Explanation; (Answer; d)
The molecular mass of CO2 (12 + 32) and N2O (28+16) = 44 amu. So gas unknown gas may be CO2 or N2O.
13. 16 g of oxygen has same number of molecules as in
(a) 16 g of CO
(b) 28 g of N2
(c) 14 g of N2
(d) 2 g of He
Explanation; (Answer; b)
According
to Avogadro’s law, equal number of moles contains equal number of molecules. 16
g oxygen constitutes its one mole. 28 g of N2 also constitutes its
one mole. So 16 g oxygen (1 mole) has the same number of molecules as in 28 g
of N2 (1 mole).
14. The volume of 3.01 x 1023 molecules of N2 gas at STP will be
(a) 3 dm3
(b) 11.2 cm3
(c) 22.4 dm3
(d) 11200 cm3
Explanation; (Answer; d)
15. The mass of one mole of electrons is:
(b) 0.55 mg
(c) 0.184 mg
(d) 1.673 mg
Explanation; (Answer; b)
Mass of 1 electron = 9.1×10−31 kg = 9.1 × 10−25 mg
1 mole of electron = 6.02 × 1023 electrons
Mass of 1 mole of electrons = mass of 1 electron × number of one mole of electron
= (9.1 × 10−25) × (6.02×1023)
= 0.54782mg ≈ 0.55mg
The mass of one mole of electrons = 0.55 mg
16. The number of moles of CO2 which contain 8.0 g of oxygen:
(a) 0.25
(b) 0.50
(c) 1.0
(d) 1.50
Explanation; (Answer; a)
Mass of 1 mole or Molar mass of CO2 = 12 x 16 x 2 = 44 g/mol
Mass of oxygen in 32 g of CO2 = 16 x 2 = 32 g
32 g of oxygen is contained in 1 mole of CO2
1 g of oxygen is contained in 1/32 mole of CO2
8 g of oxygen is contained in = 1/32 x 8 = 0.25 mole of CO2
17. The largest number of molecules are present in:
(a) 3.6 g of water
(b) 4.8 g of C2H5OH
(c) 2.8 g of CO
(d) 5.4 g of N2O5
Explanation; (Answer; a)
Hence 3.6 g water having greatest number of moles out of given options will contain largest number of molecules.
18. The formula of KAl(SO4)2 represents a total of:
Explanation; (Answer; c)
KAl(SO4)2 = 1K + 2Al + 2S + (4 x 2)O= 12 atoms
19. Which will weigh more?
(a) 0.1 mole of sucrose
(b) 1 mole of acetic acid
(c) 6.01x1023 molecules of SO2
(d) All have same
Explanation; (Answer; c)
Mass of 0.1 mole of sucrose (C12H22O11)
= nM = 0.1 x 342 = 34.2 g
Mass of 0.1 mole of acetic acid (CH3COOH)
= molar mass = 60 g
Mass of molecules of SO2 = Np/NA
x M = 6.01x1023 /6.02x1023 x 64 = 64 g
20. The number of atoms in 2.4g magnesium and 1.0 g of hydrogen are.
(a) Equal atoms
(b) Different atoms
(c) Magnesium has more atoms than hydrogen
(d) None of these
Explanation; (Answer; b)
Same number of moles contains same number
of particles. 2.4g magnesium constitutes its 0.1
mole and 1.0 g of hydrogen constitutes its 1 mole. Since moles are different,
so both of them contains different number of atoms. 1 g H has more atoms than
2.4 g Mg.
21. Objects of the size of an atom can be observed in
(a) an electron microscope
(b) atomic absorption spectrum
(c) an X-ray spectrum
(d) visible spectrum
Explanation; (Answer; a)
Objects of the size of an atom can be
observed in an electron microscope
22. Two different hydrocarbon each contain the same percentage by mass of hydrogen. It follows that they have the same
(a) Empirical formula
(b) number of atoms in a molecule
(c) number of isomers
(d) relative molecular mass
Explanation; (Answer; a)
Same percentage composition means same empirical formula.
23. % age of oxygen in combustion analysis is calculated by the formula
a) (%age of C + % of H) – 100
(b) (%age of C + % of H) +100
(c) (%age of C + % of H) 100
(d) 100 – (%age of C + % of H)
Explanation; (Answer; d)
% age of oxygen in
combustion analysis is calculated by the difference method.
24. Which one of the following has same number of electrons?
(a) F-, Na+, Ne, O2-, N3-, Mg2+
(b) Ca2+, Mg2+, Al3+, Sc3+, Ar, Ti4+
(c) F-, Cl-, I1-, Na1+, Mg2+, Ca2+
(d) All of them
Explanation; (Answer; a)
Number of electrons in cation = Z – Number
of positive charge
Number of electrons in anion = Z + Number of negative charge
25. Molecular ions are produced in mass spectrometer. Which type of molecular ions is more abundant?
(a) Positively charged
(b) Negatively charged
(c) Neutral ions
(d) Molecules cannot form ions.
Explanation; (Answer; a)
When the vaporised organic sample passes
into the ionization chamber of a mass spectrometer, it is bombarded by a stream
of electrons. These electrons have a high enough energy to knock an electron
off an organic molecule to form a positive ion. This ion is called
the molecular ion symbolized as M+. The molecular ions tend to
be unstable and some of them break into smaller fragments. All mass
spectrometers separate ions based on their mass to charge ratio, and molecules
can be ionized and analyzed just like atoms are.
26. Using the Periodic Table for the relative atomic masses, which has the least mass?
(a) 0.1 moles of silicon dioxide, SiO2
(b) Hemi moles of lithium, Li
(c) Semi moles of oxygen, O2
(d) 1.0 moles of ammonia, NH3
Explanation; (Answer; b)
Mass of 0.1 mole of SiO2 = 0.1 x 60 = 6
g
Mass of 0.5 (hemi) mole of Li = 0.5 x 7 = 3.5 g
Mass of 0.5 (semi) mole of O2 = 0.5 x 32 = 16 g
Mass of 1.0 mole of NH3 = 1.0 x 17 = 17
g
27. Which is not represented by 1mole of Nitrogen gas?
(a) 6.023 x 1023 molecules of N2
(b) 6.023 x1023 atoms of N2
(c) 12.046 x 1023 atoms of N2
(d) 28 g of N2
Explanation; (Answer; b)
One mole of N2 = molar mass = NA
molecules = 2 NA atoms
One mole of N2 = 28 g = 6.023
x1023 molecules = 2 x 6.023 x1023 (12.046 x 1023)
atoms
28. Out of 1 g of oxygen gas, 1 g of oxygen atoms and 1 g of ozone, maximum number of atoms are present in:
(a) 1 g of oxygen gas(c) 1 g of oxygen atoms
Explanation; (Answer; c)
Greater the n, greater is the number of
particles. 1 g of O atoms has largest n, so it contains maximum number of
atoms.
29. The number of atoms contained 11.2 litre of SO2 at STP are
(a) 3/2 x 6.023 x 1023
(b) 2 x 6.023 x 1023
(c) 6.023 x 1023
(d) 4 x 6.023 x 1023
Explanation; (Answer; a)
30. 2 g of oxygen contains number of atoms equal to that in
(a) 0.5 g of hydrogen gas
(b) 2.3 g of sodium
(c) 7 g of nitrogen
(d) 4 g of sulphur
Explanation; (Answer; d)
2 g of oxygen constitutes its 0.125 mole
(2/16)
0.125 mole of oxygen contains number of
atoms equal to 0.125 x 6.02 x 1023 = 7.525 x 1022 atoms
0.5 g of hydrogen constitutes its 0.25
mole (0.5/2)
2.3 g of sodium constitutes its
0.1 mole (2.3/23)
7 g of nitrogen constitutes its 0.5 mole
(7/14)
4 g of sulphur constitutes its 0.125 mole
(4/32)
4 g of sulphur and 2 g of oxygen
constitutes same number of moles (i.e. 0.125 mole), therefore, they contain
same number of atoms.
31. The largest number of molecules is in
(a) 34 g of water
(b) 28 g of CO2
(c) 46 g of CH3OH
(d) 54 of N2O5
Explanation; (Answer; a)
Greater the number of moles, greater is the number of particles. 34 g of water contains largest mole (1.88), so it contains the largest number of molecules.
(a) 34 g of water constitutes its 1.88 mole
(b) 28 g of CO2 constitutes its 1 mole
(c) 46 g of CH3OH constitutes its 1 mole
(d) 54 of N2O5 constitutes its 0.458 mole
32. 1 mol of CH4 contains
(a) 6.02 × 1023 atoms of H
(b) 3.0 g of carbon
(c) 4 g atom of Hydrogen
(d) 1.81 × 1023 molecules of CH4
Explanation; (Answer; c)
We know that 1 mole of any substance has
Avogadro number of units in it, like 1 mole of oxygen has Avogadro number of
molecules in it.
1 mole of CH4 contains 1 mole
of carbon (1 gram atom of carbon)
1 mole of CH4
contains 4 moles of hydrogen (4 gram atoms of hydrogen)
1 mole of CH4 contains 6.023 x
1023 atoms of carbon
1 mole of CH4 contains 6.023 x
1023 x 4 atoms of hydrogen (4NA atoms of H)
1 mole of CH4 contains 6.023 x
1023 x 5 atoms of C and H (5NA atoms of H)
1 mole of CH4 contains 6.023 x
1023 molecules of CH4
1 mole of CH4 contains 12 g of
carbon
1 mole of CH4 contains 4 g of
hydrogen
33. Which of the following has smallest number of molecules?
(a) 0.1 mol of CO2 gas
(b) 22 g of CO2 gas
(c) 11.21 of CO2 gas at NTP
(d) 22.4 mL of CO2 gas
Explanation; (Answer; a)
Smaller number of moles contains least number of molecules. Since CO2 has least number of mole out of given options, so it contains least number of molecules.
(a) 0.1 mol of CO2 gas
(b) 22 g of CO2 gas constitutes its 0.5 mole
(c) 11.21 of CO2 gas at NTP constitutes its 0.5 mole
(d) 22.4 mL of CO2 gas constitutes its 1 mole
34. Number of atoms in 558.5 gram Fe (atomic weight of Fe = 55.85 g mol–1) is
(a) 558.5 × 6.023 × 1023
(b) half that in 8 g He
(c) twice that in 60 g carbon
(d) 6.023 × 1022
Explanation; (Answer; c)
Number of moles in 558.5 g of Fe = 558.5/55.85 = 10 mol = 10 NA
atoms
Number of moles in 60 g of C = 60/12 = 5
mol = 5 NA atoms
35. Which of the following has least mass?
(a) 2 gram atom of nitrogen
(b) 3 x 1023 atoms of carbon
(c) 1 mole of sulphur
(d) 5.6 liter of oxygen gas at STP
Explanation; (Answer; b)
(a) 2 gram atom of nitrogen weighs 28 g (14 x 2)
(b) 3 x 1023 atoms of carbon weighs 6 g (3 x 1023 /6.02 x 1023 x 12)
(c) 1 mole of sulphur weighs 32 g
(d) 5.6 liter of oxygen gas at STP weighs 8 g.
36. One mole of
any substance contains 6.022 x 1023 atoms/molecules. Number of
molecules of H2SO4 present in 100 mL of 0.02 M H2SO4 solution
is ----------------
(a) 12.044 x 1020 molecules
(b) 6.022 x 1023 molecules
(c) 1 x 1023 molecules
(d) 12.044 x 1023 molecules
Explanation; (Answer; a)
37. 32 g of oxygen, 28g nitrogen occupy separately the volume of 22.414 dm3
(a) Size of molecules of two gases are same(b) Masses of molecules two gases are same
(c) Number of atoms of two gases are same
(d) All of these
Explanation; (Answer; c)
32 g O2 and 28 g N2 constitutes
1 mole of respective gases which contain same of molecules and also atoms (due
to same atomicity).
38. Which pair of molecules contains the same number of molecules at STP?
(a) 280 cm3 of CO2 and 280 cm3 of N2O
(b) 11.2 dm3 of O2 and 32 g of O2
(c) 44 g of CO2 and 11.2 dm3 of CO
(d) 1 gram molecule of N2 and 5.6 dm3 of Cl2
Explanation; (Answer; a)
Equal volumes of all gases have equal
number of moles and number of molecules.
39. Which of the following has the same number of molecules at STP?
(a) 1 dm3 of N2 and O2
(b) 500 cm3 of Cl2 and O2
(c) 100 cm3 of CO2 and O2
(d) All of them
Explanation; (Answer; a)
Equal volumes of all gases have equal
number of moles and number of molecules.
40. A limiting reactant is one which according to the
stoichiometric equation?
(a) has excess mass
(b) has least mass
(c) has excess number of moles
(d) has least number of moles
Explanation; (Answer; d)
A limiting reactant is one
which according to the stoichiometric equation has least number
of moles
41. In the determination of atomic ratio of the elements, the mole
ratios are divided by
(a) Least value of gram atoms of elements
(b) Atomic masses of elements
(c) Given mass of the compound
(d) molecular mass of the compound
Explanation; (Answer; a)
Atomic ratio of elements is the ratio of
moles of each element by the smallest number of moles (i.e. least value of gram
atoms) giving relative ratio of atoms in the compound
Explanation; (Answer; a)
Relative atomic mass, Ar is the ratio of
average mass of an atom of an alement to the 1/12th mass of one atom
of C – 12.
43. Which of the following has maximum mass?
(a) 0.1 gram atom of carbon
(b) Hemi mole of ammonia
(c) 6.02 x 1022 molecules of hydrogen
(d) 1120 cc of carbon dioxide at STP
Explanation; (Answer; b)
(a) 0.1 gram atom of carbon weights 1.2 g
(b) Hemi mole of ammonia weighs 8.5 g
(c) 6.02 x 1022 molecules of hydrogen weighs 0.2 g
(d) 1120 cc of carbon dioxide at STP 2.2 g
44. 0.1 mole of Na3PO4 completely dissociates in water to produce Na+
(a) 6.02 x 1022
(b) 6.02 x 1023
(c) 1.806 x 1023
(d) 1.806 x 1022
Explanation; (Answer; c)
1 mole of Na3PO4 gives total 4 moles of ions i.e. 3 moles of Na+ and 1 mole of PO4− ion.
0.1 mole of Na3PO4 gives 3 x 0.1 x NA Na+ ions = 0.3 x 6.02 x 1023 Na+ ions = 1.806 x 1023 Na+ ions
45. The largest number of H+ are produced by complete ionization of
(a) 0.1002 moles of HCl
(b) 0.051 moles of H2SO4
(c) 0.0334 moles of H3PO4
(d) All of the above
Explanation; (Answer; d)
HCl being a Monoprotic acid gives 1 mole
of H+ ion on ionization. Hence 0.102 moles of HCl gives 0.102 moles
of H+ ions.
H2SO4 being a
diprotic acid gives 2 mole of H+ ion on ionization. Hence 0.051
moles of H2SO4 gives 0.051 x 2 = 0.102 moles of H+
ions.
H3PO4
being a triprotic acid gives 3 mole of H+ ion on ionization. Hence
0.0334 moles of H3PO4 gives 0.0334 x 2 =
0.1002 moles of H+ ions.
46. 16 g of S8 contains:
(a) 6.023 x 1023 atoms of S
(b) 6.023 x 1023/8 atoms of S
(c) 6.023 x 1023/2 atoms of S
(d) 6.023 x 1023 x 0.0625 molecules of S
Explanation; (Answer; d)
We know that 1 mole of any substance has
Avogadro number of units in it, like 1 mole of oxygen has Avogadro number of
molecules in it. Sulphur has an atomic weight of approximately 32 g/mol, so the
molar mass of S8 would be 256 g/mol.
Now, 16 g of S8 would thus correspond to 0.0625 (16/256) moles and thus the number of molecules in 16 g of S8 would be 0.0625 times Avogadro’s number i.e. 0.0625 x 6.022 x 1023 molecules of S8.
47. Which of the following has the smallest number of molecules
(a) 0.1 moles of CO2
(b) 2 g of H2 at STP
(c) 16 g of O2 gas
(d) 3.4 g of NH3
Explanation; (Answer; a)
Greater is the number of moles, greater is
the number of particles and vice versa.
0.1
moles of CO2
2 g of H2 at STP constitutes
its one mole
16 g of O2 gas constitutes its
half mole
3.4 g of NH3 gas constitutes
its 0.2 mole
Since 0.1 moles of CO2 has least number of moles, so it contains the smallest number of molecules.
48. Which of the following weighs the least?
(a) 2.0 gram mole of CO2
(b) Semi mole of sucrose (C12H22O11)
(c) deci gram atom of calcium
(d) 1.5 mole of water
Explanation; (Answer; c)
(a) 2.0 gram mole of CO2 weighs 88 g (44 x 2)
(b) Semi mole of sucrose (C12H22O11) weighs 171 g (342 x 0.5)
(c) deci gram atom of calcium weighs 4 g (40 x 0.1)
(d) 1.5 mole of water weighs 27 g (18 x 1.5)
49.The largest number of molecules is present in
(a) 59 g of nitrogen peroxide
(b) 54 g of water
(c) 56 g of carbon dioxide
(d) 58 g of ethyl alcohol
Explanation; (Answer; c)Greater is the number of moles, greater is the number of particles and vice versa.
(a) 59 g of nitrogen peroxide constitutes its 0.5 mole
(c) 56 g of carbon dioxide constitutes its 1.27 mole
(d) 58 g of ethyl alcohol constitutes its 1.26 mole
50. 1g-atom of nitrogen represents
(a) 6.02 × 1023 N2 molecules
(b) 11.2 L of N2 at N.T.P.
(c) 22.4 L of N2 at N.T.P.
(d) 28 g of nitrogen
Explanation; (Answer; b)
Different equivalent of 1 g-atom of N
are
14 g N2
NA atoms of N
11.2 liter or dm3
Gram atom signifies gram atomic mass. Gram
atomic mass of N is 14 g.
At STP 1 mole of nitrogen gas (N2)
occupies 22.4 liters of N2 gas
So 14 g of nitrogen occupies 11.2 liters of N2 gas.
51. Number of atoms in 560 g of Fe (atomic mass 56 g/mol) is
(a) is twice that of 70 g of N
(b) is half as 2 grams of hydrogen
(c) Is half that of 20 g H
(d) None of these
Explanation; (Answer; a)
Number of atoms in 560 g of Fe = mass/molar mass = 560/56=10 mole
Number of atoms in 70 g of N = mass/molar mass = 79/14 =5 mole
52.Compared to 88g of carbon dioxide, 88g of propane contains
(a) The same number of atoms
(b) The same number of molecules
(c) Less atoms
(d) More molecules
Explanation; (Answer; b)
Both propane
and carbon dioxide have same molar mass of 44 g/mol. For same masses, compounds
with same molar mass have same number of moles and consequently same number of
molecules.
53. The incorrect statement for 14 g of CO is
(a) It occupies 2.24 liter at STP
(b) It corresponds to 0.5 mol of CO
(c) It corresponds to same mole of CO and N2
(d) It corresponds to 3.01 x 1023
Explanation; (Answer; a)
14 g of CO occupies 11.2 liter at STP
14 g of CO corresponds to 0.5 mol of CO
14 g of CO corresponds to same mole of CO and N2
14 g of CO corresponds to 3.01 x 1023
54. 10 moles of H2O contains
(a) 100 moles of bonds
(b) 25 moles of hydrogen bonds
(c) 30 atoms
(d) 100 moles of electrons
Explanation; (Answer; d)
10 moles of H2O contains 20 moles of bonds (2 x 10)
10 moles of H2O contains 40 moles of hydrogen bonds (4
x 10)
10 moles of H2O contains 30 atoms (3 x 10)
10 moles of H2O contains 100 moles of electrons (10 x
10)
55. The maximum number of molecules is present in
(a) 15 L of H2 gas at STP
(b) 1.5 g of H2 gas
(c) 5 L of N2 gas at STP
(d) 5 g of O2 gas
Explanation; (Answer; b)
(a) 15 L of H2 gas at STP constitutes its 0.669 moleGreater number of moles corresponds to greater number of molecules (also atoms for same atomicity). Here 1.5 g of H2 gas has greatest number of moles and hence it contains greatest number of molecules.
56. Which among the following is the heaviest?
(a) one mole of oxygen
(b) 100 amu of uranium
(c) One molecule of sulfur trioxide
(d) 44 g carbon dioxide
Explanation; (Answer; d)
(a) one mole of oxygen weighs 32 g
(b) 100 amu of uranium weighs 3.95 x 10-20 g
(c) One molecule of sulfur trioxide weighs 1.32 x 10^-22 g
(d) 44 g carbon dioxide
57. If proton number of two atoms is same then it can be concluded that
(a) They are isotopes
(b) Their compounds will be similar in reactivity towards other compounds
(c) Both have same colours
(d) Both have same melting point
Explanation; (Answer; b)
Isotopes have same chemical properties
58. A mole of any substance is related to
(a) Number of particles
(b) Mass of a substance
(c) Volume of gaseous substances
(d) All of these
Explanation; (Answer; d)
59. This one of the following pairs has the same number of molecules
(a) 10 g H2 and 10 g CH4
(b) 10 g H2 and 50 g CH4
(c) 10 g H2 and 16 g CH4
(d) 10 g H2 and 80 g CH4
Explanation; (Answer; c)
Same number of moles contains same number of molecules.
(a) 10 g H2 and 10 g CH4 constitutes 5 mole
H2 and 0.625 mole CH4
(b) 10 g H2 and 50 g CH4 constitutes 5 mole
H2 and 3.125 mole CH4
(c) 10 g H2 and 16 g CH4 constitutes 5 mole
H2 and 1 mole CH4
(d) 10 g H2 and 80 g CH4 constitutes 5 mole
H2 and 5 mole CH4
60. How many moles of chlorine atoms are present in 0.99 g of C2H4Cl2?
(a) 0.01
(b) 0.0178
(c) 0.02
(d) 0.75
Explanation; (Answer; c)
61. One mole of carbon-12 has a mass of:
(a) 0.012 kg
(b) 0.0224 kg
(c) 12 kg
(d) 1 kg
Explanation; (Answer; a)
Mass of one mole of carbon-12 is 12 g or 12/1000 = 0.012 kg
62. How many moles are present in 52 g of aspartame (C14H18N2O5)?
(a) 0.177
(b) 0.36
(c) 1.2
(d) 0.54
Explanation; (Answer; a)
Molar
mass of aspartame (C14H18N2O5) =
(12 x 14) + (18x1) + (14x2) + (16x5) = 294 gmol−1
No. of
moles (n) = m/M = 52/294 = 0.177 mole
63. The number of moles of CO2 which contain 16 g of oxygen
(a) 0.50
(b) 1.0
(c) 0.25
(d) 1.50
Explanation; (Answer; a)
1 mole
(44g) of CO2 contains 12 g carbon (1mole) and 32 g (2mole) oxygen
32 g
oxygen is present in 1 mole of CO2
16 g
oxygen is present in =16/32 mole of CO2 = 0.50 mole
64. A vessel contains 10 g of N2, 10 g of H2 and 10 g O2. Which one has the largest number of atoms?
(a) H2
(b) N2
(c) O2
(d) All of these
Explanation; (Answer; a)
Molar
masses of N2, h2
and O2 are 28, 2 and 32 respectively. Greater the molar mass lesser
is the number of moles and lesser is the number of molecules and number of
atoms (for same atomicity). Hence the number of their moles are in their order
H2 > N2 > O2 in their equal masses. The
same order follows for number of molecules and number of atoms.
65. What is total volume of 0.5 g of H2, 16 g of O2 and 7.0 g of N2 present in a mixture at STP?
(a) 2.4 dm3
(b) 0.224 dm3
(c) 22.4 dm3
(d) 11.2 dm3
Explanation; (Answer; c)
Species
……………. H2 : O2 : N2
Given
masses ……… 0.5 16 7.0
Molar
masses ……… 2 32 28
No of moles
………… 2/2 16/32 7.0/28
Total
moles …………. 0.25 + 0.5
+ 0.25 = 1 mole
Total
volume ……….. 1 mole = 22.4 dm3
66. The mass of one mole of electrons in mg is:
(a) 1.008 mg
(b) 1.673 mg
(c) 0.55 mg
(d) 0.184 mg
Explanation; (Answer; c)
Mass of 1 electron = 9.1 X 10−³¹
kg.
1 mole = 6.023 X 10²³
So 1 mole of electrons
has a mass of = 9.1 x 10−³¹ x 6.023 x 10²³ ≈ 5.481 x 10−7 kg
≈ 5.481 x 10−4 g ≈ 5.481 x 10−1 mg ≈ 0.5481 or
0.55 mg
67. The mass of one mole of electrons is:
(a) 1.008 mg
(b) 0.184 mg
(c) 1.673 mg
(d) 0.55 mg
Explanation; (Answer; d)
Mass of
one electron is 9.11 x 10−31 kg
Mass of
one mole (6.02 x 1023) electron is = 9.11 x 0−31 x 6.02 x
1023 = 54.84 x 10−8 kg or 5.5 x 10−7
kg
Mass of one mole of electron in mg = 5.5 x 10−7 kg x 1 x 106 = 5.5 x 10−1 mg or 0.55 mg
68.The number of moles of CO2 which contain 8.0 g of oxygen:
(a) 0.25
(b) 0.50
(c) 1.0
(d) 1.50
Explanation; (Answer; a)
32 g O =
1 mole of CO2
8.0 g O =
1/32 x 8.0 = 0.25 mole of CO2
69. One mole of hydrogen gas at s.t.p. contains how many atoms?
(a) 6.02 x 1023
(b) 3.01 x 1024
(c) 1.2 x 1024
(d) 12.04 x 1046
Explanation; (Answer; c)
No of atoms = atomicity x n x NA = 2 x 1 x NA = 2NA = 2 x 6.02 x 1023 =1.2 x 1024
70. The mass of a single hydrogen atom is:
(a) 1.67x10−20 g
(b) 1.67x10−24 g
(c) 1.67x10−27 g
(d) 1.67x10−23 g
Explanation; (Answer; b)
Mass of
single atom = atomic mass/NA = 1/6.02 x 1023 = 1.66 x 10−24
g
71. 1.12 dm3 of nitrogen gas at STP weighs:
(a) 28 g
(b) 2.8
(c) 1.4
(d) 14
Explanation; (Answer; c)
22.4 dm3
of nitrogen gas at STP weighs 28 g
11.2 dm3
of nitrogen gas at STP weighs 11.2/22.4 x 28 g = 1.4 g
72. The mass of one atom of coal is:
(a) 1.99 x10−22 g
(b) 1.99 x10−23 g
(c) 1.99 x10−24 g
(d) 1.99 x10−20 g
Explanation; (Answer; b)
Mass of single atom = atomic mass/NA = 12/6.02 x 1023 = 1.99 x 10−23 g
73.The weight of one mole of KAl(SO4)2.12H2O is:
(a) 574g g
(b) 584 g
(c) 474 g
(d) 684 g
Explanation; (Answer; c)
The
weight of one mole of KAl(SO4)2.12H2O is equal
to its gram formula mass as it is an ionic compound.
KAl(SO4)2.12H2O
= 39 + 27 + 2(32) + 8(16) + 12(18) = 474 g
74. The density of oxygen gas at STP is:
(a) 1.43 g/dm3
(b) 2.43 g/dm3
(c) 2.74 g/dm3
(d) 0.42 g/dm3
Explanation; (Answer; a)
Density of gas = molar mass/molar volume = 32/22.4 = 1.43 gdm-3
75. What is the mass of one mole of bromine molecule?
(a) 80 g
(b) 160 g
(c) 35 g
(d) 70 g
Explanation; (Answer; b)
Molecular
mass of bromine (Br2) = 80 x 2 = 160 g
76. The volume occupied by a hemi (0.1) mole of a gas at stp is:
(a) 22400 cm3
(b) 5.6 dm3
(c) 2.24 dm3
(d) 22.4 dm3
Explanation; (Answer; c)
Volume of
gas at STP = n x molar volume = 0.1 x 22.4 = 2.24 dm3 or 2240 cm3
or 0.00224 m3
77. The number of protons in one molecule of HNO3 are:
(a) 7
(b)8
(c)24
(d)32
Explanation; (Answer; c)
No of
protons in HNO3 = 1 + 7 + 3(8) = 32
78. One cm3 oxygen gas at stp contains about:
(a) 1 x 1020 atoms
(b) 6 x 1023 atoms
(c) 1.67 x 1024 atoms
(d) 0.53 x 1019 atoms
Explanation; (Answer; d)
No. of
atoms = atomicity x (Vg/VM) x NA = 2 (1/22400)
NA = 8.93 x 10-5 NA = 8.93 x 10-5 x
6 x 1023 = 5.37 x 1018 0.537 x 1019
79. The relative atomic mass of chlorine (Cl) is 35.5 amu, the mass in gram of 0.5 moles of chlorine gas is:
(a) 71 g
(b) 35.5 g
(c) 142 g
(d) 17.75 g
Explanation; (Answer; b)
Mass = nM
= 0.5 x 71 = 35.5 g
80. If 28 g of N2 gas comprises of z molecules; how many molecules are there in 16 g of oxygen?
(a) z
(b) 2 z
(c) ½ z
(d) ¼ z
Explanation; (Answer; c)
28 g of N2
gas constitutes its one mole which contains NA (here z) molecules.
16 g of O2 constitutes its half mole which contains ½ NA
molecules (i.e. ½ z).
81. What is the relative molecular mass, Mr, of CuSO4.5H2O?
(a) 127
(b) 160
(c) 178
(d) 249.5
Explanation; (Answer; d)
relative
molecular mass = Sum of atomic masses of all atoms
relative
molecular mass CuSO4.5H2O = 63.5 + 32 + (16 x 4) + 5(18)
= 249.5 amu
82. The total number of ions in FeCl3 is
(a) 6.02 x 1023
(b) 12.04 x 1023
(c) 18.06 x 1023
(d) 24.08 x 1023
Explanation; (Answer; d)
total
number of ions in 1 mole = total ions x n x NA = 4 x 1x NA=
4NA = 4 x 6.02 x 1023 = 24.08 x 1023 or 2.408
x 1024
83. Which of the following has maximum number of atoms?
(a) 1.8 g of H2O
(b) 8.8 g of CO2
(c) 16 g of O2
(d) 12 g of CH4
Explanation; (Answer; d)
No of
atoms from mass = mass/molar x atomicity x NA
No of
atoms 1.8 g of H2O = 1.8/18 x
2 x NA = 0.2 NA
No of
atoms 8.8 g of CO2 = 8.8/44 x
3 x NA = 0.6 NA
No of
atoms 16 g of O2 = 16/32 x
2 x NA = 1 NA
No of
atoms 12 g of CH4 = 12/16 x
5 x NA = 3.75 NA
84. The number of moles of CO2 which contains 8.0 g of oxygen?
(a) 0.25
(b) 0.50
(c) 1.0
(d) 1.50
Explanation; (Answer; a)
32 g of
oxygen is present in 1 mole of CO2
1 g of
oxygen is present in 1/32 mole of CO2
8 g
oxygen is present in 1/32 x 8 mole of CO2 = 0.25 mole of
CO2
85. An atom has 8 protons and 10 neutrons, the atom is:
(a) N
(b) B
(c) O
(d) Ne
Explanation; (Answer; c)
Atomic
number characterized an element. O has an atomic number of 8.
86.The number of protons in one molecule of H2SO4 is:
(a) 40
(b) 50
(c) 60
(d) 30
Explanation; (Answer; b)
Total
number of protons = Sum of number of protons of all atoms
Total
number of protons in H2SO4 = (1 x 2) + 16 + (4 x 8) = 50
87. ----------- is a generic name for hydrogen cations; protons, deuterons and tritons.
(a) Nucleons
(b) Hydride
(c) Hydrocarbons
(d) Hydron
Explanation; (Answer; d)
The
generic name for three types of hydrogen cations i.e. protons, deuterons and
tritons is Hydron.
88. The number of atoms present in 0.5 moles of Nitrogen atoms is same as in:
(a) 12 g of C
(b) 32 g of S
(c) 24 g of Mg
(d) 8 g of O
Explanation; (Answer; d)
8 of O constitutes
its half mole.
89. How many moles of helium gas occupy 22.4 litre at 0ºC and 1 atm pressure?
(a) 0.11
(b) 1.0
(c) 0.90
(d) 1.11
Explanation; (Answer; b)
At STP
(0ºC temperature and 1 atm pressure), one mole of all gases occupy a constant
volume of 22.4 litre which is called molar gas volume.
90. The total number of protons in 10 g of calcium carbonate is
(a) 1.5057 x1024
(b) 2.0478 x 1024
(c) 3.0115 x1024
(d) 4.0956 x1024
Explanation; (Answer; c)
Protons
in 1 mole of CaCO3 = Z of Ca + Z of C + Z of O x 3 Þ 20 + 6+24 = 50 moles of protons
100 g of
CaCO3 contains 50 moles of protons
1 g of
CaCO3 contains 50/100 moles of protons
10 of
CaCO3 contains = 50/100 x 10 x 6.02 x 1023 moles of
protons = 3.0115 x 1024 protons
91. The formula of hydrogen phosphate of
certain metal is MHPO4. The formula of metal sulphate would be
(a)
MSO4 (b) M2SO4 (c) M3SO4
(d) M2(SO4)3
Explanation; (Answer; a)
The net
charge on HPO4 ion is 2-. Thus in MPHO4, the charge on M
would be 2+. The charge of SO4 is 2-, so formula of metal sulphate
(M) would be MSO4.
92. If NA is Avogadro’s number, then number of valence electrons in 4.2 g of nitride ion (N3−) is
(a) 2.4
(b) 4.2
(c) 1.6
(d) 3.2
Explanation; (Answer; a)
N has 5
valence electrons. It gains 3 electrons to form nitride ion (N3−) which
now contains total 8 valence electrons.
14 g of
nitride ion contains valence electrons = 8 NA
1 g of
nitride ion contains valence electrons =
8NA/14
4.2 g of
nitride ion contains valence electrons = (8NA/14) x 4.2 = 2.4 NA
93. Vapour density of a gas is 22. What is its molecular mass?
(a) 33
(b) 44
(c) 22
(d) 11
Explanation; (Answer; b)
Molar
mass = 2 x vapour density = 2 x 22 = 44
94. The numerical value of ‘N/n’ (where N is the number of molecules in a given sample of gas and ‘n’ is the number of moles of the gas) is
(a) 8.314
(b) 0.0821
(c) 1.62×10−24
(d) 6.02×1023
Explanation; (Answer; c)
6.023
×1023 molecules (N) = 1 mole (n)
∴ N/n = 1/6.023 × 1023 =
1.62×10−24
95. The number of sulphur atoms present in 0.2 moles of S8 molecules is
(a) 9.63 × 1022
(b) 4.82 × 1023
(c) 1.20 × 1023
(d) 9.63 × 1023
Explanation; (Answer; d)
No of
atoms = atomicity x n x NA= 8 x 0.2 x NA= 1.6 NA
= 1.6 × 6.02 × 1023
= 9.632 × 1023
96. What is the number of atoms in 0.1 mol of a tetra-atomic gas? (NA= 6.02 x 1023 molecules)
(a) 6.022 x 1022
(b) 6.022 x 1023
(c) 2.4088 × 1022
(d) 2.4088 × 1023
Explanation; (Answer; d)
One mole
of any substance contains 6.022 x 1023 particles (Avogadro's number).
Thus
Number of
molecules = number of moles x Avogadro's
number
Number of molecules= 0.1 x 6.022 x 1023 = 6.022 x 1022
Each molecule of tetra-atomic gas contains 4 atoms (atomicity = 4), therefore,
Number of atoms in = atomicity x NA = 4 x 6.022
x 1022 = 24.088 × 1022 OR 2.4088 × 1023
97. 1 atom of an element weighs 1.792 × 10−22 g. The Atomic mass of the element is
(a) 17.92
(b) 1.192
(c) 108
(d) 64
Explanation; (Answer; c)
1 atom of
an element weighs 1.792 × 10−22
g
6.02 × 1023
atom of an element weighs = 1.792 × 10−22 × 6.02 × 1023
= 107.878 ≈ 108 g
98. A sample of phosphorus trichloride (PCl3) contains 1.4 moles of the substance. How many atoms are there in the sample?
(a) 4
(b) 5.6
(c) 8.431 x 1023
(d) 3.36 x 1024
Explanation; (Answer; d)
One mole
of any substance contains 6.022 x 1023 particles (Avogadro's number)
Thus
Number of
molecules = number of moles x Avogadro's
number
Number of
molecules = 1.4 × 6.022 × 1023 = 8.431
x 1023
Each molecule of PCl3 contains 4 atoms; 1 P atom and 3 Cl atoms, therefore,
Number of atoms in 1.4 mole PCl3 = 8.431 × 1023
× 4 = 3.36 × 1024
99. The mass of 1 atom of hydrogen is
(a) 0.5 g
(b) 1.6 × 10−24 g
(c) 1 g
(d) 3.2 × 10−24 g
Explanation; (Answer; b)
Mass of 1
atom = atomic mass/NA = 1/6.02
× 1023 =
1.6 × 10−24 g
100. Two containers of the same size are filled separately with H2 gas and CO2 gas. Both the containers under the same T and P will contain the same
(a) Number of electrons
(b) Number of molecules
(c) Weight of gas
(d) Number of atoms
Explanation; (Answer; b)
Since
volume of container is same, so both gases have same volume and same no of
moles and consequently same number of molecules.
101. The number of atoms in 4.25 g of NH3 is approximately
(a) 1 × 1023
(b) 2 × 1023
(c) 4 × 1023
(d) 6 × 1023
Explanation; (Answer; d)
Number of
atoms in a molecule = atomicity x (mass/M) x NA = 4 x (4.25/17) x NA
= 1NA = 6 × 1023
102. One mole of any covalent compounds contains same number of
(a) Atoms
(b) Molecules
(c) Ions
(d) Electrons
Explanation; (Answer; b)
covalent
compounds consist of discrete molecules.
103. One atom of an element X weighs 6.664 ×10–23 g. The number of gram atoms in 40 kg of it are
(a) 10
(b) 10000
(c)20
(d) 1000
Explanation; (Answer; d)
Gram atomic mass = mass of 1 mole atoms × mass of one atom
= (6.022×1023) × (6.664 ×10−23) = 40.01 g
Gram atoms
= moles = mass/molar mass = 40000/40 = 1000
∴ The number of gram atoms in
40 kg = 1000
104. Which one of the following is not an iso-electronic pair?
(a) Ca+, K+
(b) Na+, Ne
(c) Ca2+, Cl−
(d) O2−, Al3+
Explanation; (Answer; a)
Iso-electric
ions have same number of electrons.
Number of
electrons in Ca+ = 20 – 1 = 19
Number of
electrons in K+ = 19 – 1 =
18
105. The mass of CO containing the same amount of oxygen as in 88 g of CO2 is
(a) 56 g
(b) 28 g
(c) 112 g
(d) 14 g
Explanation; (Answer; c)
Molar mass of CO2 = 44 g
Mass of oxygen in 44 g of CO2
= 32 g
Mass of oxygen in 88 g of CO2
= 64 g
Molar mass of CO = 28 g
Mass of O in CO = 16 g
16 g of O = 30 g CO
64 g of O = (28/16) x 64 = 112 g
106. The mass of CO containing the same number of oxygen atoms as are present in 88 g of CO2 is
(a) 56 g
(b) 28 g
(c) 112 g
(d) 14 g
Explanation; (Answer; c)
No. of molecule = (m/M) NA
= (88/44) x 6.02 x 1023 = 1.2046 x 1024 molecules
No of atoms of O = atomicity x no of molecules = 2 x 1.2046 x 1024 = 2.4092 x 1024 atoms
6.022 x 1023 atoms of O = 28g of CO
Mass = Np/NA x M = 2.4092 x 1024 /6.022 x 1023 x 28 = 112 g
Alternate Method
Molecular mass of CO2 =
12 + 2 x 16 = 44
1mol of CO2 = 44 g
44 g of CO2 contain =
6.022 x 1023 molecules
88 g of CO2 contain =
6.022x1023 x 88/44 = 12.046 x 1023 molecules
Each molecule of CO2
contain 2 oxygen atoms
No. of oxygen atoms in 12.046 x 1023 molecules of CO2 = 2 x 12.046 x 1023 = 2.4092 x 1024
Now, we have to calculate the mass
of CO containing 2.4092 x 1024 atoms of oxygen.
Mass of CO containing 6.022 x 1023
oxygen atoms = 28 g.
Mass of CO containing 2.4092 x 1023
oxygen atoms = 28/6.022 x 1023
x 2.4092 x 1024 = 112 g
107. Number of O2 molecules present in one litre flask at pressure 7.6 × 10–10 mm of Hg at 0ºC are
(a) 2.69 × 1010
(b) 2.69 × 1011
(c) 2.69 × 109
(d) None of these
Explanation; (Answer; a)
PV = nRT Þ PV = Np/NA
RT Þ Np = PVNA/RT
Np = (7.6 × 10–10
/760 atm) (1 liter) (6.02 x 1023 molecules-mol-1)/(0.0821atm-liter-mol-1
K-1 )x (0+273 K)
Np = 1 x 10-12 x 6.02 x 1023/5.733
Np = 2.69 × 1010
molecules
108. Volume occupied by one molecule of water (density = 1 g cm–3) is
(a) 9.0 × 10–23 cm3
(b) 6.023 × 10–23 cm3
(c) 3.0 × 10–23 cm3
(d) 5.5 × 10–23 cm3
Explanation; (Answer; c)
Density
of water (ρ) = 1
Molar
mass of water (M) = 18 g mol–1
Density = Molar mass/molar volume Þ Molar volume = molar mass/density = 18/1 = 18 cm3 mol–1
6.02 x 1023
molecules of water = 18 cm3
1
molecules of water = 18/6.02 x 1023 = 2.989 x 10–23 or 3
x 10–23 cm3
109. The diameter of atoms is of the order of
(a) 2 x 10−5 m
(b) 2 x 10−10 m
(c) 2 x 10−2 m
(d) 2 x 10−3 m
Explanation; (Answer; b)
The atomic size or atomic
radius is of the order of 10−7cm or 10−9m or 1
nanometer (nm).
ratom ≈10−10 m
rnucleus ≈10−15 m
Under most definitions the radii of
isolated neutral atoms range between 30 and 300 pm (trillionths of
a meter), or between 0.3 and 3 ångströms. Therefore, the radius of an atom is more than 10,000 times
the radius of its nucleus (1–10 fm), and less than 1/1000 of the wavelength of visible light (400–700 nm).
110.One mole of ethane and one mole of ethanol have an equal
(a) Number of molecules
(b) Number of electrons
(c) Number of atoms
(d) Masses
Explanation; (Answer; c)
(a) 2.5 x 1020
(b) 5.0 x 1010
(c) 2.5 x 1012
(d) 5.0 x 1030
Explanation;
(Answer; c)
1
nanogram (ng) of uranium = 10−9 g
No. of
atoms = n x NA = (m/M) x NA = (10−9/238)x 6.02
x1023 = 2.5 x 1012 atoms of uranium
112. How many moles of electrons are found in 401.4 g of P3−?
(a) 194
(b) 402
(c) 154
(d) 232.2
Explanation;
(Answer; d)
1 mole of P3−contains 30.97 g of P3−. Atomic number is equal to number of electrons.
The electronic configuration of
phosphorus P (Z=15) is 1s2 2s2 2p6,3s2
3p3 showing that it has 15 electrons. Phosphorus, P gains three
electrons and converts into P3− giving total 18 electrons.
No of moles in in 401.4 g of P3−
= 401.4/30.97 = 12.96 mole
1 mole of P3− ion
contains 18 electrons
12.96 mole of P3− ion
contains 18 x 12.96 electrons = 233.2 mole of electrons
113.Which one of the following will have largest number of atoms?
(a) 1 g Au(s)
(b) 1 g Na(s)
(c) 1 g Li(s)
(d) 1 g of Cl2(g)
Explanation;
(Answer; c)
114. 6.3 g of HNO3 has mass
of nitrate ions
(a) 6.2 g
(b) 62 g
(c) 0.62 g
(d)
3.1g
Explanation;
(Answer; a)
Molar
mass of HNO3 = 63 g
Molar
mass of nitrate (NO3−) ion = 62 g
63 g HNO3
contains 62 g nitrate ion
6.3 g HNO3
contains (62/63) x 6.3 nitrate ion = 6.2 g nitrate ions
115. 9 g of ice has number of covalent bonds
(a) 6.02 x 1023
(b) 3.01 x 1023
(c)1.661 x 10-23
(d) 1.505 x 1023
Explanation;
(Answer; a)
Molar
mass of ice (H2O) = 18 g
18 g ice
contains = 2 mole covalent bonds (H–O–H)
9 g ice
contains (2/18) x 9 mole covalent bonds = 1 mole of covalent bond = 6.02 x 1023
covalent bonds
116. A
well-known ideal gas is enclosed in a container having volume 5603 cm3
at STP. Its mass comes out to be 16 g. The unknown gas is
(a) O2
(b) CH4
(c) SO2
(d)
CO2
Explanation;
(Answer; c)
5603 cm3
of gas at sTP weighs 16 g
22400 cm3
of gas at STP weighs (16/5603)x 22400 (molar mass) = 63.96 ≈ 64 g
The molar
mas of SO2 is 64.
117. Which of the following has the same
atomic and molecular mass?
(a) bromine
(b) oxygen
(c) neon
(d)
nitrogen
Explanation;
(Answer; )
118. The wavelength of visible light is
500 nm. In SI unit this value is
(a) 500 x 10−9 m
(b) 5 x 10−9 m
(c) 500 x 10−7 m
(d)
5 x 10−8 m
Explanation;
(Answer; a)
1nm = 10−9 m
500 nm = 500
x10−9 m = 5 x 102 x 10−9 = 5 x 10−7
m
119. Which of the following contains the
least numbers of molecules?
(a) 1 g of H2
(b) 4 g of O2
(c) 2 g of N2
(d)
3 g of Cl2
Explanation;
(Answer; d)
Substance
containing least number of moles would have least number of molecules. Here 3g
of Cl2 contains least number of moles (0.042), so it has least
number of molecules.
120. 1 gram molecule of any gas at STP
occupies the volume of
(a) 22.4 cm3
(b) 0.0224 dm3
(c) 22400 cm3
(d)
100 cm3
Explanation;
(Answer; c)
1 gram
molecules constitutes one mole of that substance. 1 mole of any gas at STP
occupies the fixed volume known as molar gas volume which is equal to 22.4 dm3
or 22400 cm3 or 0.0224 m3.
121. The number of moles of carbon in 200 g
of CaCO3 is
(a) 1 mole
(b) 2 mole
(c) 3 mole
(d)
4 mole
Explanation;
(Answer; b)
100 g of
CaCO3 contains 1 mole of C
200 g of
CaCO3 contains 1/100 x 200 = 2 mole of C
122.The volume of semi-mole of a gas at STP is
(a) 11.2 cm3
(b) 11200 cm3
(c) 22.4 ml
(d) 0.0224 m3
Explanation;
(Answer; b)
Semi mole
means half mole. One mole of any gas STP occupies 22.4 dm3. Hence
half mole of any gas occupies half of 22.4 dm3 i.e. 11.2 dm3.
But this is not given in the options. 11.2 dm3 equals to 11200 cm3.
So correct option is b.
123. At NTP, 5.6 L of gas weighs 8 grams.
The vapour density of a gas is
(a) 32
(b) 40
(c) 16
(d)
8
Explanation;
(Answer; c)
We know
that NTP is Normal Temperature and Pressure which is defined as a temperature
of 200C and 1 atmosphere. Vapor density is defined as the
density of a vapor in relation to that of hydrogen. Vapor density can tell us
whether a gas is denser or less dense than air.
124.The vapour densities of two gases are
in the ratio of 1:3. Their molecular masses are in the ratio of:
(a) 1:3
(b) 1:2
(c) 2:3
(d)
3:1
Explanation;
(Answer; a)
125. An unknown gas has a density 1.96 gL−1.
Then gas is
(a) CO
(b) NO2
(c) CO2
(d)
SO2
Explanation;
(Answer; c)
Volume of
1 mole of gas at STP = 22.4 L
126. 2.2 g of an unknown gas is present in
a container of 1.12 L. Then unknown gas is
(a) CO2
(b) CO
(c) N2O
(d) Both
a and c
Explanation;
(Answer; d)
The
molecular mass of CO2 (12 + 32) and N2O (28+16) = 44 amu.
So gas unknown gas may be CO2 or N2O.
127. 16 g of oxygen has same number of
molecules as in
(a) 16 g of CO
(b) 28 g of N2
(c) 14 g of N2
(d) 2 g of He
Explanation;
(Answer; b)
According
to Avogadro’s law, equal number of moles contains equal number of molecules. 16
g oxygen constitutes its one mole. 28 g of N2 also constitutes its
one mole. So 16 g oxygen (1 mole) has the same number of molecules as in 28 g
of N2 (1 mole).
128. The volume of 3.01 x 1023
molecules of N2 gas at STP will be
(a) 3 dm3
(b) 11.2 cm3
(c) 22.4 dm3
(d)
11200 cm3
Explanation;
(Answer; d)
129. 8.8 g of CO2 contains …………
atoms
(a) 0.4 NA
(b) 0.2 NA
(c) 0.1 NA
(d)
None of them
Explanation;
(Answer; b)
44 g of
CO2 contains 3NA atoms
8.8 g of
CO2 contains 3NA x 8.8/44 = 0.2 NA atoms
130. The number of atoms in 11.2 L of SO2
gas at STP is
(a) NA/2
(b) 3NA/2
(c) 3NA
(d)
NA
Explanation;
(Answer; b)
No. of
atoms = V/VM x NA x atomicity = 11.2/22.4 NA x
3 = 3NA x ½ = 3NA /2
131.The total number of electrons present
in 18mL of water is
(a) 6.02 x 1022
(b) 6.02 x 1023
(c) 6.02 x 1024
(d)
6.02 x 1025NA
Explanation;
(Answer; c)
The total number of electrons present in 18 ml of water is
6.023 × 1024.
Number of electron in one molecule of H2O is 2 + 8 = 10.
Density = 1 g/ml
∴18 ml means 18 g
Moles =18 / 18 = 1
Molecules = 6.02 × 1023
Number of Electrons = 6.02 × 1023 × 10 =
6.02 × 1024
132. The volume of 1.0 g of hydrogen at NTP
is
(a) 2.24 L
(b) 22.4 L
(c) 1.12 L
(d)
11.2 L
Explanation;
(Answer; d)
1 mole of
any gas at NTP occupies 22.4 L.
1 g of
hydrogen (H2) constitutes its half mole.
Half mole
of any gas at NTP occupies half of 22.4 L i.e. 11.2 L
133. 7.5 grams of a gas occupy 5.6 litres
of volume at STP. The gas is
(a) NO
(b) N2O4
(c) CO
(d)
CO2
Explanation;
(Answer; a)
Molar
mass = (Vm / Vg) x mass = 22.4/5.6 x 7.5 = 44 g
Molar
mass of NO = 30 gmol-1
134. How many moles are there in m3
of any gas at NTP?
(a) 44.6
(b) 4.46
(c) 4.46 x 10−5
(d)
0.0446
Explanation;
(Answer; a)
No. of
moles of gas at STP = Vg/VM = 1 m3/0.0224 m3/mol
= 44.6 mole
135. no.
of moles in 28 kg of silicon.
(a) 0.1
(b) 0.01
(c) 1000
(d)
10
Explanation;
(Answer; c)
No. of
moles = mass/molar = 28 x 103 g/28 = 103 or 1000 mole.
136. no.
of moles in 1000 mg of helium is
(a) 0.25
(b) 0.01
(c) 25
(d)
0.2
Explanation;
(Answer; a)
No. of
moles = mass/molar = 1000 ÷ 103 g/4 = ¼ mole or 0.25 mole
137. 49g of phosphoric acid dissolved in
excess quantity of water, it will yield ___________ moles of hydrogen ion (H+)
___ moles of phosphate (PO43–)
(a) 1.5, 02
(b) 1.5, 01
(c) 03, 01
(d)
1.5, 0.5
Explanation;
(Answer; d)
Molar
mass of phosphoric acid (H3PO4) = 3+31+64 = 98 gmol−1
98g (1
mole) of phosphoric acid (H3PO4) ionizes to give 3 mole H+
ions and 1 mole phosphate ion (PO43–)
49 (0.5
mol) of phosphoric acid (H3PO4) ionizes to give 1.5 mole
H+ ions and 0.5 mole phosphate ion (PO43–)
138. Number of atoms present in 22.414 liter
of hydrogen gas at STP
(a) 3.01x1023
(b) 2.408x1023
(c) 6.02x1023
(d)
1.204x1024
Explanation;
(Answer; d)
No of
atoms in given volume of gas at STP = atomicity x Vg/Vm)NA= 2 x
(22.414/22.414)NA = 2 x 1 x NA = 2NA
= 2 x
6.02x1023 = 1.204x1024
139. Number of molecules in 18ml of water
will be almost
(a) 18x6.02x1023
(b) 55.6x6.02x1023
(c) 6.02x1023
(d) 2x6.02x1023
Explanation;
(Answer; c)
Molecular weight of H2O =
18 g
Because density of water = 1 g/mL
18 g water = 18 ml water
∵ 18 ml
of H2O contain = 6.02×1023 molecules
of H2O
140). 0.8 moles of ammonia and 0.8 moles of
methane gas have.
(a) Same no. of molecules
(b) Different no of molecules
(c) Different volume
(d) Same no. of atoms
Explanation;
(Answer; a)
Same
number of moles contains same number of molecules (and aslo same number of
atoms for same atomicity).
141. 14g of silicon and 7g of nitrogen
contains:
(a) Nitrogen contains more atoms
(b) Si contains more atoms
(c) Same atoms
(d) Different atoms
Explanation;
(Answer; c)
14 g of
silicon and 7g of nitrogen both constitutes their half moles. Same number of
moles contains same number of particles.
142. One mole of CO2 contains:
I) 6.02x1023 atoms of carbon
II) 6.02x1023 atoms of oxygen
III) 1gram molecule of CO2
IV) Molecules having 22.4dm3 volume
(a) I and III
(b) II and III
(c) II, III and IV
(d) I, III and IV
Explanation;
(Answer; d)
One mole
of CO2 contains 1gram molecule of CO2
One mole
of CO2 contains NA (6.02x1023) molecules of CO2
One mole
of CO2 contains 22.4dm3 volume of CO2
One mole
of CO2 contains 1 gram atom or 1 mole of C
One mole
of CO2 contains 2 gram molecule or 2 mole of O
One mole
of CO2 contains NA (6.02x1023) atoms of C
One mole
of CO2 contains NA (6.02x1023) molecules of O2
143. 180 g of glucose contains atoms of
carbon
(a) 0.5NA
(b) 3NA
(c) 6NA
(d) 12NA
Explanation;
(Answer; d)
180 g of
glucose constitutes its 1 mole
1 mole
glucose = 6 moles of C = 6NA
atoms of C
1 mole
glucose = 12 moles of H = 12NA atoms of H
1 mole
glucose = 6 moles of C = 6NA
atoms of O
144. No. of ions in one formula unit of sodium chloride
(a) 3 x 6.02 x1023
(b) 2
(c) 3
(d) 4
Explanation;
(Answer; b)
one
formula unit of sodium chloride (NaCl) contains 2 mole of ions or 2NA
ions (2 x 6.02 x 1023 ions).
145.One gram formula of Na2CO3
has the number of ions than the number of formula units when dissolved in
water.
(a) Twice
(b) Thrice
(c) quadrice
(d) Same
Explanation;
(Answer; b)
One gram
formula or 1 mole of gives total three moles of ions (2 Na+ and 1 CO32−)
on ionization.
146. The no. of moles of CO2 which contains 6g of carbon
(a) 0.25
(b) 0.50
(c) 1.0
(d) 1.50
Explanation;
(Answer; b)
12 g
carbon = 1 mole of CO2
6 g
carbon = 1/12 x 6 = 0.5 mole of CO2
147. no. of gram ions in 3.9g K+
(a) 6.02x1022
(b) 0.1
(c) 1
(d)
0.01
Explanation;
(Answer; a)
No of
gram ions or mole = mass/molar mass x NA = 3.9/39 x NA = 0.1NA = 0.1 x 6.02x1022
= 6.02x1023
148. no. of formula units in 95g MgCl2.
(a) 6.02x1023
(b) 12.04x1023
(c) 18.06x1023
(d) One
Explanation;
(Answer; a)
No of
formula units or mole = mass/molar mass x NA = 95/95 x NA = 1NA= 6.02x1023
149.no. of gram atoms of oxygen in 48g
ozone
(a) 1
(b) 2
(c) 3
(d)
18.06x1023
Explanation;
(Answer; a)
No of
gram atoms or mole = mass/molar mass = 48/48 x NA = 1
150. 16 gram of methane contains
___________ no. of electrons
(a) 6.02x1023
(b) 6.02x1024
(c) 6.02x1025
(d)
6.02x1026
Explanation;
(Answer; b)
16 g of
methane (CH4) = 1 mole
1 mole of
methane (CH4) contains total 6+ 4(1) = 10 moles of electrons = 10NA
electrons = 10 x 6.02x1023 = 6.02x1024
151. 60g CO32– and
24g Mg+2 have number of ions.
(a) Same
(b) Different
(c) CO32– has more
(d) Mg+2
has more
Explanation;
(Answer; a)
No of
moles in 60g CO32– = mass/molar mass = 60/60 = 1 mole
No of
moles in 24g Mg+2 =
mass/molar mass = 24/24 = 1 mole
Same
number of moles contain same number of particles.
152. A molecule of haemoglobin is made up
of nearly
(a) 10,1000 atoms
(b) 50,000 atoms
(c) 2500 atoms
(d)
1500 atoms
Explanation;
(Answer; d)
153.Each molecule of haemoglobin is 68000
times heavier than one atom of
(a) C
(b) H
(c) N
(d)
O
Explanation;
(Answer; b)
Each
molecule of haemoglobin is 68000 times heavier than one atom of H.
154. Relative atomic mass of an element is
the mass of an element relative to
(a) 1/12 mass of carbon-12
(b) 1/12 mass of carbon
(c) 1/16 mass of oxygen
(d) None of them
Explanation;
(Answer; a)
Relative
atomic mass of an element is calculated by comparing it with 1/12th
mass of one atom of standard C-12.
45. The relative atomic mass of chlorine
is 35.5. What is the mass of 2 moles of chlorine gas?
(a)142 g
(b) 71 g
(c) 35.5 g
(d)
18.75 g
Explanation;
(Answer; a)
Mass from
moles = n x M = 2 x 71 = 142 g
156. How many moles of hydrogen atoms does 3.2 g of methane (CH4) contain?
(a) 0.02
(b) 0.2
(c) 0.4
(d) 0.8
Explanation;
(Answer; a)
16 g of
CH4 contains 4 moles of H
3.2 g of
CH4 contains 4/16 x 3.2 = 0.8 mole
157. One mole of C2H5OH
contains the number of H-atoms
(a) 6.02 x 1023
(b) 3.61 x 1024
(c) 1.81 x 1024
(d)
6.02 x 1024
Explanation;
(Answer; b)
There are
6 mole of H atoms in 1 mole of C2H5OH
So, there
will be 6NA atoms or 3.61 x 1024 atoms of H in 1 mole of C2H5OH
158. 3.01 x 1022 Ag+
ions is present in
(a) 85 g AgNO3
(b) 0.85 g AgNO3
(c) 8.5 g AgNO3
(d)
18.5g AgNO3
Explanation;
(Answer; c)
3.01 x 1022
Ag+ ions constitutes its 0.05 mole.
6.02 x 1023
Ag+ ions = 170 g (1 mole) of AgNO3
3.01 x 1022
Ag+ ions = 3.01 x 1022 /6.02 x 1023 x 170 g (1
mole) of AgNO3 = 8.5 g AgNO3
159. The mass of one mole of proton is
(a) 1.008 g
(b) 0.184 g
(c) 1.673 g
(d)
1.008 mg
Explanation;
(Answer; a)
Mass of 1
proton = 1.672×10−24g
Mass of
one mole (6.02 x 1023) protons = 1.672×10−24 ×
6.022×1023 = 1.0068784 g ≈ 1.007 g
160. 0.5 mole of
CH4 and 0.5 mole of SO2 gases have equal
(a) Total number of atoms
(b) Number of molecules
(c) Mass in grams
(d) volume
Explanation;
(Answer; b)
For same
number of moles of different substances, there are same number of molecules.
161. A beaker containing 180 g of water contains:
(a) 6.02 x 1023 molecules
(b) 6.02 x 1024 molecules
(c) 10.02 x 1025 molecules
(d) 12.01 x 1023 molecules
Explanation; (Answer; b)
180 g of water constitutes its 10 moles.
No of molecules in 10 moles = n NA = 10NA = 10 x 6.02 x 1023 = 60.2 x 1023 = 6.02 x 1024 molecules
162. The present scale of atomic mass is based on 1 amu being equal to the mass of:
(a) 1 hydrogen atom
(b) 1/10 of O-16
(c) 1/12 of C-12 atom
(d) 1/16 of O-16
Explanation; (Answer; c)
An atomic mass unit (abbreviated as a.m.u) is a physiochemical constant and it is defined as one twelfth (1/12) of the mass of a single atom (the most abundant lightest isotope) of carbon-12 (12C). [Because the mass of an atom’s electrons is negligible compared with the mass of its proton and neutrons, defining 1 amu as 1/12 the mass of a 12C atom means that both protons and neutrons have a mass of almost exactly 1 amu]. 1 amu is equivalent to 1.66 x 10–24 g OR 1.66 x 10–27 kg (which is approximately equal to the mass of one hydrogen atom). Prior to 1960, the amu was defined in terms of the mass of a 16O-isotope (1.6599 x 10–27 kg).
163. Chlorine atom and chloride ions
(a) Have same number of protons
(b) Are allotropes of chlorine
(c) Have same number of electrons
(d) Are chemically identical
Explanation; (Answer; a)
Atom and its ion have same number of protons but different number of electrons. Atom and its ion are chemically different.
164.This one of the following pairs has the same number of molecules
(a) 10 g H2 and 10 g CH4
(b) 10 g H2 and 50 g CH4
(c) 10 g H2 and 16 g CH4
(d) 10 g H2 and 80 g CH4
Explanation; (Answer; c)
Same number of moles contains same number of molecules.
(a) 10 g H2 and 10 g CH4 constitutes 5 mole H2 and 0.625 mole CH4
(b) 10 g H2 and 50 g CH4 constitutes 5 mole H2 and 3.125 mole CH4
(c) 10 g H2 and 16 g CH4 constitutes 5 mole H2 and 1 mole CH4
(d) 10 g H2 and 80 g CH4 constitutes 5 mole H2 and 5 mole CH4
1. To determine the molecular formula of glucose, its empirical formula is multiplied by:
2. The prefix 1018
3. What is the ratio of the weight of water formed to the weight of oxygen used in the formation of water?
4. One mole of carbon-12 has a mass of:
5. What is the mass of one mole of iodine molecule?
6. Which of the following is classified as a monoatomic element?
7. The present scale of atomic mass is based on 1 amu being equal to the mass of:
8. The mass of a single hydrogen atom is:
9. 11.2 dm3 of nitrogen gas at STP weighs:
10.The mass of one atom of coal is:
1. Solution
For glucose, value of integer n is 6.
2. Solution
Prefixes (Multiples & Fractions) used with S.I. and Metric Units
|
Prefixes |
Multiples |
Prefixes |
Fractions |
| Dec(D) |
101 (Ten) |
deci (d) |
10–1 |
| Hecto(H) |
102 (Hundred) |
centi (c) |
10–2 |
| kilo (k) |
103 (Thousand) |
milli (m) |
10–3 |
| Mega(M) |
106 (Million) |
micro (µ) |
10–6 |
| Giga (G) |
109 (Billion) |
nano (n) |
10–9 |
| Tera (T) |
1012 (Trillion) |
pico (p) |
10–12 |
| Peta (P) |
1015 (Quadrillion) |
femto(f/fm) |
10–15 |
| Exa (E) |
1018 (Quintillion) |
atto (a) |
10–18 |
| Zetta (Z) |
1021 (Sextillion) |
zepto (z) |
10–21 |
| Yottta (Y) |
1024 (Septillion) |
yacto (y) |
10–24 |
3. Solution
4. Solution
Mass of one mole of carbon-12 is 12 g or 12/1000 = 0.012 kg
5. Solution
6. Solution
Noble gases (e.g. He, Ne, Ar, Kr, Xe and Rn) are classified as monoatomic elements.
7. Solution
An atomic mass unit (abbreviated as a.m.u) is a physiochemical constant and it is defined as one twelfth (1/12) of the mass of a single atom (the most abundant lightest isotope) of carbon-12 (12C).
[Because the mass of an atom’s electrons is negligible compared with the mass of its proton and neutrons, defining 1 amu as 1/12 the mass of a 12C atom means that both protons and neutrons have a mass of almost exactly 1 amu].
1 amu is equivalent to 1.66 x 10–24 g OR 1.66 x 10–27 kg (which is approximately equal to the mass of one hydrogen atom). Prior to 1960, the amu was defined in terms of the mass of a 16O-isotope (1.6599 x 10–27 kg).
8. Solution
9. Solution
22.4 dm3 of nitrogen gas at STP weighs 28 g
10. Solution
11. Solution
The weight of one mole of KAl(SO4)2.12H2O is equal to its gram formula mass as it is an ionic compound.
KAl(SO4)2.12H2O = 39 + 27 + 2(32) + 8(16) + 12(18) = 474 g
12. Solution
13. Solution
14. Solution
32 g of oxygen is present in 1 mole of CO2
1 g of oxygen is present in 1/32 mole of CO2
8 g oxygen is present in 1/32 x 8 mole of CO2 = 0.25 mole of CO2
15. Solution
Mass of one electron is 9.11 x 10-31 kg
Mass of one mole (6.02 x 1023) electron is = 9.11 x 0-31 x 6.02 x 1023 = 54.84 x 10‒8 kg or 5.5 x 10‒7 kg
Mass of one mole of electron in mg = 5.5 x 10-7 kg x 1 x 106 = 5.5 x 10‒1 mg or 0.55 mg
16. Solution
8 of O constitutes its half mole.
17. Solution
18. Solution
Protons in 1 mole of CaCO3 = Z of Ca + Z of C + Z of O x 3 Þ 20 + 6 + 24 = 50 moles of protons
100 g of CaCO3 contains 50 moles of protons
1 g of CaCO3 contains 50/100 moles of protons
10 of CaCO3 contains = 50/100 x 10 x 6.02 x 1023 moles of protons = 3.0115 x 1024 protons
19. Solution
2 g of oxygen constitutes its 0.125 mole
0.125 mole of oxygen contains number of atoms equal to 0.125 x 6.02 x 1023 = 7.525 x 1022 atoms
0.5 g of hydrogen constitutes its 0.25 mole
2.3 g of sodium constitutes its 0.1 mole
7 g of nitrogen constitutes its 0.5 mole
4 g of sulphur constitutes its 0.125 mole
4 g of sulphur and 2 g of oxygen constitutes same number of moles (i.e. 0.125 mole), therefore, they contain same number of atoms.
20. Solution
MCQs on Mole Concept Set # 3