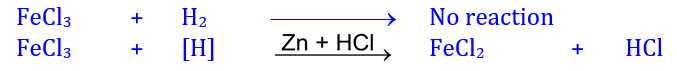Position of Hydrogen in
Periodic Table
Basis of
Classification of Elements
Elements are
arranged in the periodic table on the basis of their electronic configuration and to some
extent on the basis of their properties.
Resemblance
of Hydrogen with Elements of Different Groups
The
classification of hydrogen has been an issue throughout the history of the
periodic table. Hydrogen is the first element of the periodic table and its
position is anomalous. Hydrogen symbolized as
(1). It resembles
alkali metals with respect to electronic configuration, electropositive character,
valency, oxidation state, reducing behaviour, combination with electronegative
elements and liberation at cathode.
(2). It resembles
halogens with respect to electronic
configuration (one electron less than the nearest
noble gas configuration), ionization energy, electronegative character,
oxidation state, diatomic nature and liberation at anode.
(3). It resembles
carbon family with respect to electronic
configuration (half-filled valence shell), thermodynamic properties, non-metallic character, variable oxidation state, reducing behaviour, formation of covalent compounds and close association with organic compounds.
Comparison of Hydrogen with Alkali
Metals
It resembles alkali metals with respect to electronic configuration, electropositive
character, valency, oxidation state, combination with
electronegative elements, reducing behaviour, cation formation,
hydration of cations in water and liberation at cathode.
Comparison of Hydrogen with Carbon
Family
Comparison of Hydrogen with Halogens
It resembles
halogens with respect to electronic configuration, ionization energy,
electronegative character, oxidation state, diatomic nature (atomicity) and
liberation at anode.
Comparison of Hydrogen with Alkali
Metals
It resembles alkali metals with respect to electronic configuration, electropositive
character, valency, oxidation state, combination with
electronegative elements, reducing behaviour, cation formation,
hydration of cations in water and liberation at cathode.
Similarities of Hydrogen with Alkali metals
1. Same number of Valence Electron or Outer
Electronic Configuration
Both hydrogen and alkali metals have one electron in valence
shell. Electronic structure of H is 1s1
and that of alkali metals is ns1.
2. Same Valency and
Oxidation State
Both are monovalent and show +1
oxidation state.
3. Formation of Cation
Like alkali metals, hydrogen forms
mono-positive ion (H+) by losing one electron.
4. Hydration of Cations in water
Like alkali metal cations (Li+
and Na+) H+ ion is hydrated in aqueous solution.
H+ + H2O → H3O+
5. Collection at Cathode
Both are collected at cathode during electrolysis.
6. Reducing Behaviour
Both are reducing agents.
7. Great Affinity for
Non-metals giving Analogous Compounds
Both are electropositive and both have a great affinity for
non-metals (especially halogens) to form electrolytes which are analogous.
H2 + X2 →
2HX (Hydrogen
halides)
2Na+ X2 →2NaX (Alkali
metal halides)
8. Same Behaviour of their Halides in Water
Both
hydrogen halides (HX) and alkali metal halides (MX) ionize in aqueous solution
to yield positive ions such as H+ and alkali metal ions (M+)
respectively.
HX(aq) ⇌ H+(aq) +X– (aq)
MX(aq) ⇌ M+(aq) + X– (aq)
9. Lack of Acting as a Central Atom
Both hydrogen and alkali metal cannot
act as a central atom in ternary compounds.
10. Lack of Forming Multiple Bonds
Both hydrogen and alkali metal cannot
act form multiple bonds.
11. metallic
Behaviour of Solid Hydrogen under High Pressure
The most
valid argument for placing hydrogen in group IA is that under very high
pressure, hydrogen has the properties of a metal.(It has been argued, that any
hydrogen present at the center of the Planet Jupiter is likely to be a metallic
solid). Finally, hydrogen combines with a handful of transition metals to form
materials that behave as if they were alloys of two metals.
Dissimilarities of Hydrogen with Alkali metals
1. Difference in State and
Nature
Hydrogen is a gaseous non-metal while
alkali metals are metallic solid.
2. Difference in Valence Shell Completion
Hydrogen needs one electron to complete its valence shell whereas
alkali metals need seven electrons to complete their valence shell.
3. Half Filled Valence Shell
Hydrogen has half-filled valence shell
while alkali metals do not.
4. Difference in Atomicity
It exists as diatomic molecule (H2),
alkali metals exist in monoatomic form.
5. Difference in Bonding
It forms covalent (HCl) as well as ionic
compounds (Na+H–) whereas alkali metals form only ionic
compounds.
6. Formation of Anion by
Hydrogen
Hydrogen can form uni-negative hydride
(H–) ion but alkali metals do not form anions.
7. Difference in Stability
of Cations
H+ ion is unstable but Na+,
K+ etc are stable.
8. Instability of Hydrogen
Cation
Unlike Na+, K+ ions, H+ ions do
not exist free in aqueous solution except in the solvated form such as H3O+
or H9O4+[i.e. H+(H2O)4].
9. Difference in Nature of
Oxides
Oxide of hydrogen (H2O) is
neutral whereas the oxides of alkali metals (Na2O, K2O)
are basic.
10. Difference in Thermodynamic
Properties
Thermodynamic properties such as ionization potential (1312 kJmol–1
or 13.6 eV), electron affinity (–72.8 kJmol–1) and electronegativity
(2.1) of hydrogen are quite high to those of alkali metals (I.P values = 3.9-5.4
eV), E.N = 0.7-1.0).
H(g) → H+(g) + e– I.P = +1312 kJ mol–1
K(g) → K+(g) + e– I.P = + 418 kJ mol–1
11. Exhibition of Variable Oxidation States by
Hydrogen
Hydrogen exhibits variable oxidation states of +1, –1 or even zero
but alkali metals show only fixed oxidation states of +1.
12. Collection at Anode
Hydrogen is
collected at anode during electrolysis of molten ionic metallic hydrides while
alkali metals are always collected at cathode.
13. Oxidizing Behaviour of Hydrogen
Hydrogen
acts as an oxidizing agent when combines with highly reactive s-block
elements.
Comparison with Carbon Family
Similarities of Hydrogen with Carbon Family
1. Same
Nature of Valence Shell (half-filled Valence Shell)
Both
hydrogen and elements of carbon family have half-filled valence shell. In case of hydrogen half duplet (one
electron, K1) and in case of carbon half octet (four electrons, L4).
2. Same
Elemental’s Nature
Both are
typical non-metals.
3. Reducing
Behaviour
Both are reducing agent.
4. Formation
of Covalent Compounds
Hydrogen forms covalent compounds (e.g. H– Cl) like members
of group IVA (e.g C≡O, O=C=O).
5. Formation
of Anions
Hydrogen can form anions (H–) like C4–.
6.Close
Association with Organic Compounds
Both hydrogen and carbon are found in close
association with organic compounds.
7. Same
Thermodynamic Properties
Thermodynamic
properties such as ionization potential, electron affinity and
electronegativity of hydrogen are almost similar to those of group IVA
elements.
8. Bad
Conductivity
Liquid hydrogen is bad conductor of electricity, so is
carbon, except graphite.
9. Same
Number of Isotopes
Both hydrogen and carbon has 3 isotopes.
10. Exhibition
of Variable Oxidation States
Both shows
positive and negative oxidation states. i.e. both show variable oxidation
states (in case of hydrogen +1, -1, 0 and in case of elements of carbon family
+4, +2, +1, -1, -2, -4).
Dissimilarities of
Hydrogen with Carbon Family
1. Difference
in State
Hydrogen is gas while Carbon Family’s elements
are solids.
2. Difference
in Atomicity
Hydrogen exists as diatomic molecule (H2),
but members of group IVA as monoatomic form.
3. Difference
in Valency
Hydrogen is monovalent showing monovalency
while group IVA members show tetravalancy.
4.Difference
in Valence Electrons and Valence Shell
Hydrogen has one electron in valence shell
consisting of s-orbital requires only one electron to complete its valence
shell (K) while group IVA elements have 4 electrons in valence shell comprising
of ‘s’ and p-orbitals and needs 4 electrons to fulfill its valence shell.
5.Lack
of Forming Anions by Later Congeners of Group IVA
Hydrogen forms anion (H– ion), but elements of carbon family cannot form anion except C (C4–, C22–).
6. Lack
of Showing Allotropy by Hydrogen
Hydrogen does not exhibit allotropy, but group
IVA elements do exhibit allotropy.
7. Lack of Forming Multiple Bonds
Hydrogen cannot form multiple bonds but carbon
of group IVA can form multiple bonds.
8. Lack of Acting as a Central Atom
Hydrogen
cannot be a central atom but carbon family elements frequently act as a central
atom.
Comparison with Halogens
Similarities of Hydrogen with Halogens
1 Similarity
in Valence Shell Completion
Both require one electron to get
respective inert gas configuration. Thus H– and F– ions
are comparable.
H + e– → H– [Same as He, 1s2]
F
+ e– → F– [Same
as Ne, 1s2, 2s2 2p6]
2. Same
Elemental’s Nature and State
Both are typical non-metals and gas
at S.T.P. (except Bromine and Iodine).
3. Same
Valency and Oxidation State
Both are univalent and exhibiting –
1 oxidation state.
4. Same
Atomicity
Both exist as diatomic form e.g. H2
like F2, Cl2.
5. Formation
of Covalent Compounds
Hydrogen preferably forms covalent
compounds with non-metals (CH4, SiH4) so do halogens (CCl4,
SiCl4).
6. Formation
of Anions of Similar type
Like halogens (which form uninegative halide ion X– like F–,
Cl– ions), hydrogen can form H– ion by the gain of an
electron from electropositive metals.
Thus Na+H– and Na+Cl– are
comparable.
7. Great Affinity for metals
giving Analogous Compounds
Both are
electronegative in nature and both have a great affinity for non-metals
(especially halogens) to form electrolytes (ionic metal hydrides and metal
halides) which are analogous. Thus Na+H–
and Na+Cl– are comparable.
H2 + X2
→2HX (Hydrogen halides)
2Na + X2 →2NaX (Alkali metal halides)
8. Formation
of Isomorphous Compounds
Both alkali metal hydrides (NaH)
and alkali metal halides (NaCl) are Isomorphous having cubic structure.
9. Close
Association with Organic Compounds
Both are closely associated with
organic compounds.
10. Lack
of Forming Multiple Bonds
Both hydrogen and halogens cannot
form multiple bonds.
11. High
I.P
Hydrogen has very high value of I.P (13.6 eV) which is
comparable to that of I.P value of halogens (13.0 eV)
Dissimilarities of Hydrogen with
Halogens
1. Difference
in nature of Valence Shell and Valence Electron
Hydrogen has one electron in valence shell which consists of s-orbital
(1s1) while group VIIA elements have 7 electrons in valence shell
which consists of s and p orbitals (ns2 np5).
2. Difference
in State and Colour
H2 is colourless gas,
but halogens are coloured gases.
3. Difference
in Collection during Electrolysis
H2 collects at cathode
while halogens at anode during electrolysis.
4. Lack
of Forming Cation by halogens
H2 forms H+
ion; halogens do not form cation.
5. Difference
in Nature of Oxide
Oxide of hydrogen (H2O)
is neutral but oxides of halogens (Cl2O7) are acidic.
6. Difference
in Electron Affinity
Electron affinity of hydrogen is
much less than halogens.
7. Difference
in Redox Behaviour
H2 is reducing agent,
but halogens are oxidizing agent.
8. Instability
of Hydride Anion
H– ion is unstable while X– ions are stable. H–
ion is incapable of existence in water because it reacts with H2O to
liberate H2 gas immediately but X– ions do not react with
H2O.
H– + H2O → H2 + OH–
Unlike typical halides ions, the overall
enthalpy of formation of hydride ion is endothermic:
9. Difference
in Covalency
The maximum covalency of hydrogen
is only 1 while that of halogens is 7.
10 Lack
of Acting as Central Atom
Hydrogen cannot be central atom but
halogens are frequently act as a central atom.
Separate
Position of Hydrogen
1. Hydrogen forms neutral oxide of (H2O). It is neither acidic like oxides of
halogens nor
basic like alkali metals oxides.
2. Moreover,
hydrogen has a unique atomic structure (having singly positive charge single proton bearing nucleus lacking
neutron around which single electron revolves in K-shell whose maximum capacity
is 2 electrons).
It is, therefore, justifiable that hydrogen should not be confined or associated with any particular group like alkali metals or halogens rather it should be allotted a separate position or a special place in a box of its own detached from the
main body of the periodic table.
Conclusion on Position of Hydrogen in
Periodic Table
(1) It is difficult to decide where hydrogen belongs in the periodic table because of its unique physical properties.
For example, the first I.P of hydrogen (1312 kJ/mol) is roughly halfway between the elements with the largest 2372 kJ/mol) and the smallest (376 kJ/mol) ionization energies. Similarly hydrogen has and electronegativity (2.1) midway between the extremes of the most electronegative fluorine (E.N=4.0) and the least electronegative Cs (E.N=0.7) elements. On the basis of electronegativity, it is tempting to classify it as a semi-metal.
(2). It does not
have metallic characteristics at ordinary temperature and pressure but under
very high pressure, it is expected to behave like a metal.
(3) H+ has
very small size (~1.5 × 10–3 pm) as compared to normal atomic and
ionic sizes of 50 to 220 pm. It cannot exist freely and is always associated
with other atoms or molecules.
In the light of above discussions and facts,
it is evident that hydrogen resembles as well as differs from elements of group
IA, IVA and VIIA. Hence its exact
position in Periodic Table still remains undecided. It is
generally and controversially placed with group IA elements due to similar
electronic configuration.
Summary Position of Hydrogen
Hydrogen has electronic configuration 1s1.
On one hand, its electronic configuration is similar to the outer electronic
configuration (ns1) of alkali metals of group IA of the periodic
table. Hydrogen, therefore, has resemblance to alkali metals, which lose one
electron to form uni-positive ions. Like alkali metals, hydrogen forms oxides,
halides and sulphides. However, unlike alkali metals, it has a very high
ionization enthalpy and does not possess metallic characteristics under normal
conditions.
On the other hand, like halogens (with
ns2 np5 configuration belonging to the VIIA group of the
periodic table), it is short by one electron to the corresponding noble gas
configuration, helium (1s2).
It has resemblance with halogens, which gain one electron to form uni-negative
ion. in terms of ionization
enthalpy, hydrogen resembles more with halogens, I.P of F is 1680 kJ mol–1
and that of H is 1312 kJ mol–1. Like halogens, it forms a diatomic
molecule, combines with elements to form hydrides and a large number of
covalent compounds. However, in terms of reactivity, it is very low as compared
to halogens.
Atomic Hydrogen and
Nascent Hydrogen
Atomic Hydrogen
It is the
hydrogen in the atomic form (H) formed by reduced pressure thermal
decomposition or by electrical dissociation of ordinary molecular hydrogen (H2)
symbolized as H. Hydrogen obtained by
dissociation of molecular hydrogen is known as atomic hydrogen. Hence atomic
hydrogen is isolated hydrogen.
Langmuir ,in 1915,
obtained atomic hydrogen by dissociating on a hot filament of tungsten or
platinum. The dissociation of molecular hydrogen is an endothermic process.
The atomic
hydrogen is stable only for a fraction of a second and is extremely reactive.
It is obtained by passing dihydrogen gas at atmospheric pressure through an
electric arc struck between two tungsten rods. The electric arc maintains a
temperature around 4000 - 4500°C.
As the
molecules of dihydrogen gas pass through the electric arc, these absorb energy
and get dissociated into atoms as
This arrangement
is also called atomic hydrogen torch.
Nascent Hydrogen
It is the
hydrogen in the atomic form at the time of its generation from a chemical
reaction denoted as [H]. It is highly reactive. It is prepared by the action of
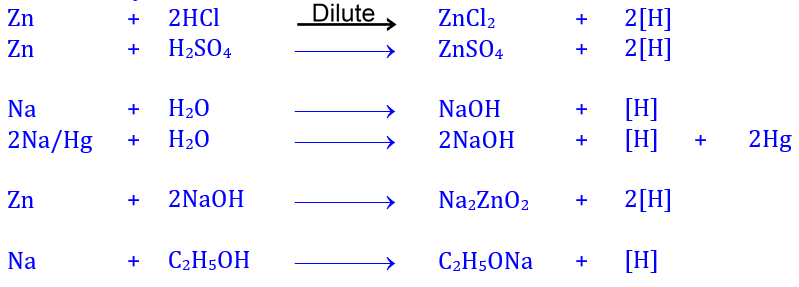
dilute HCl/H2SO4 on zinc, or by the action of water on
sodium amalgam or by treating sodium with ethanol.
The term nascent hydrogen is used to call hydrogen that is liberated
during a chemical reaction. It is considered that hydrogen liberated during the
progression of a chemical reaction is initially in the atomic state; it is then
combined to form molecular hydrogen and released as hydrogen gas (or else, this
atomic hydrogen will react with some other available ions).
The hydrogen
gas prepared in the reaction mixture in contact with the substance with which
it has to react, is called nascent hydrogen. It is also called newly born
hydrogen. It is more reactive than ordinary hydrogen. For example, if ordinary
hydrogen is passed through acidified KMnO4 (pink in
colour), its colour is not discharged. On the other hand, if zinc pieces are
added to the same solution, bubbles of hydrogen rise through the solution and
the colour is discharged due to the reduction on KMnO4 by
nascent hydrogen.
Stability
The life
period of atomic hydrogen is only 1/3rd
of a second but can be
extended under special circumstances to 10
seconds.
Properties of Atomic (Nascent) Hydrogen
1.It is obtained
from diatomic ordinary hydrogen only under extreme conditions because very high
bond energy of 104 kcal (435 kJ mole–1) is needed to dissociate
covalently bonded ordinary hydrogen.
2. It is more
energetic and more reactive than ordinary hydrogen. That is why its reactions
take place at ordinary temperature even below it, because like H2
gas it does not require 104 kcal mole–1 to break the covalent bonds.
Due to its hyper reactivity, it is highly short lived as it readily combines
itself to form molecular hydrogen.
Similarities Between Atomic Hydrogen and
Nascent Hydrogen?
1. Both
are isolated atomic states of hydrogen.
2. Both
species are highly reactive and energetic.
Atomic Hydrogen is more reactive than Molecular Hydrogen
Atomic (nascent) hydrogen is much more reactive than ordinary molecular
hydrogen. That is why, its reactions take place at ordinary temperature or even
below it. Due to its hyper reactivity, it is highly short-lived as it readily
combines itself to form molecular hydrogen (H2). The high reactivity
of atomic hydrogen is explained by considering that it is in atomic form and
possesses extra energy.
Atomic hydrogen is chemically much more reactive than molecular hydrogen
because unlike strong covalently bonded molecular hydrogen (H–H) which requires
a very high bond dissociation energy of 435 kJ/mol (104 kcal/mole) to break its
H–H bond into atomic hydrogen before they react, atomic hydrogen is available
in atomic form having high energy and hence its atoms being more reactive are
ready at once for the reaction to proceed and there is no need to supply 435
kJ/mol of energy to the reaction. Thus atomic hydrogen undergoes reaction
readily and vigorously under ordinary temperature or even below it or even with
those compounds which do not react with molecular hydrogen.
Evidence of High
Reactivity from Chemical Reactions
The high chemical reactivity of nascent hydrogen than molecular hydrogen
is visualized by their reactions with as acidic ferric chloride solution. When
H2 gas is passed through brownish colour acidic ferric chloride
solution, no appreciable change is observed. But when a piece of zinc metal is
added in the acidified FeCl3 solution, nascent hydrogen is generated
[by the reaction of zinc and HCl (acid present in solution)] which reduces
brown FeCl3 into greenish colour ferrous chloride.
The mechanism of this reaction is given
below:
Uses of Atomic Hydrogen
1. It is a powerful
reducing agent.
2. It is used to
prepare Atomic Hydrogen Torch (AHT) to attain a temperature of 4000–5000°C
which is employed in welding purposes. Heat produced by Atomic Hydrogen Torch
is not by burning hydrogen but from recombination of hydrogen atoms releasing
bond energy. Metals like Pt, Pd etc accelerate this recombination.
Reactions of Atomic Hydrogen
1. Addition
Reactions with Metals and Formation of Ionic Hydrides
2. Addition
Reactions with Non-Metals and Formation of Covalent Hydrides
3.Reducing
Action on Metallic oxides, chlorides and Unsaturated Organic Compounds
1. Addition
Reactions with Metals and Formation of Ionic Hydrides
2. Addition
Reactions with Non-Metals and Formation of Covalent Hydrides
3. Reducing
Action on Metallic oxides, chlorides and Unsaturated Organic Compounds
It is powerful reducing agent as it tends to lose its single valence
electron to change into H+ (of compounds) attaining +1 oxidation
state thereby reducing metal oxides and metal chlorides to metal while itself
oxidizes to H2O and HCl respectively. It also reduces unsaturated
organic compounds to saturated organic compounds.
(a)Reduction of
Metal Oxides to Metal (Displacement reactions with Oxides)
(b) Reduction of
Metal Chlorides to Metal (Displacement reactions with Chlorides)
(c) Reduction
of Unsaturated Organic Compounds to Saturated compounds
(Hydrogenation)
Isotopes of Hydrogen
Definition
The existence of isotopes of elements was first discovered by J.J.
Thomson in 1913. The name of isotope
was introduced (assigned) by Soddy because they have the same atomic
number and hence occupied the same place in the periodic table. (Isotope is a
Greek word; iso = same; topos = place). Nearly all elements found in nature are
mixture of several isotopes. [There are 287 different isotopic species
in nature].
“Isotopes are atoms
of the same element having same atomic number but different mass numbers
(atomic masses). In other words isotopes
are different forms of atoms of an element which have same number of protons
(and also electrons) but different number of neutrons in their respective
nuclei”.
Different isotopes of an element have same chemical properties due
to their identical electronic configuration (i.e. same number of electrons in
the shells) but they have different physical properties because of their different
atomic masses.
Out of 92
natural elements, 23 elements have no isotopes, each consisting of only one
kind of atoms. [It is strictly improper to refer to elements that exist in only
one atomic form as having “one isotope”; actually such elements as Be, F, Na, Al,
P, Sc, Mn, Co, As, Y, Nb, Rh, Cs, Pr, Tb, Ho, Tm, Bi etc have no isotopes i.e.
they have no other atomic form that is like them in all respects except mass. The
term isotope requires the existence of at least two elemental forms, in the
same sense that the word twin requires the existence of a pair. Recently the
term mono-isotopic is evolved for elements found in nature as a single atomic
or isotopic form]. The remaining 69 natural elements have 2 to 10 isotopes
each.
The heavier
isotopes of elements usually occur very rarely in the atomic population (e.g. 1
part in 4500 for 2H, 1 part in 140 for U-235; in the exceptional
case of chorine, the ratio of isotopes 35 and 37 is about 3 to 1).
Of these isotopes, only tritium is radioactive and emits low energy
b– particles (t_, 12.33 years).
Since the isotopes have the same
electronic configuration, they have almost the same chemical properties. The
only difference is in their rates of reactions, mainly due to their different
enthalpy of bond dissociation. However, in physical properties these isotopes
differ considerably due to their large mass differences.
Isotopic Forms of Hydrogen
Hydrogen
exists in three isotopic forms
1. Protium.
2. Deuterium.
3. Tritium.
Summary of Characteristics of Isotopes of Hydrogen
1. Protium
1. It is the simplest isotope and it is
just ordinary hydrogen.
2. It is symbolized as 1H1
having one proton in the nucleus and one electron in 1s orbital and no neutron.
It is the only isotope of
hydrogen having more proton than neutron.
3. It is the most abundant isotope of
hydrogen with an abundance of 99.88% [i.e. naturally free occurring hydrogen contains about 99.88%
Protium].
2. Deuterium
1. It is the natural heaviest isotope
of hydrogen and hence also known as Heavy Hydrogen.
2. It is represented as 1H2
having one neutron in the nucleus in addition to one proton and one electron in
K-shell. It is the only
isotope of hydrogen having same number proton, neutron and electron.
3. It has an abundance of 0.0156% of
terrestrial hydrogen (in the ratio of one atom of deuterium to 6000 atoms of ordinary hydrogen i.e.
1:6000 (1:1500 in book).
4. It is used as a moderator in fission
power rectors to slow down neutrons.
3. Tritium
1. It is the artificial radioactive isotope
of hydrogen with half life of 12.5 years.
2. It is symbolized as 1H3
having two neutrons in addition to one proton in the nucleus and one electron
in K-shell. It is the only
isotope of hydrogen having more neutron than proton.
3. It has a very minute abundance of 4
x 10–15% (4 x 10–50% in some books) [in the ratio of one
atom of tritium to every
1018 atoms of ordinary hydrogen i.e. 1: 1018 (1:107
in some books)].
4. It is formed in the environment by
cosmic ray bombardment.
5. It is used in thermonuclear weapons,
fusion reactions, in making hydrogen or fusion bomb, in making luminous paints and as a tracer.
Heavy Water/Dueteride
Deuterium
reacts with oxygen to form Deuterium Oxide (D2O) which is commonly
called Heavy Water due to being 1.1 times heavier than ordinary water. Heavy
water or deuterium oxide or dueteride is a binary compound of deuterium or
heavy hydrogen with oxygen formulated as .It is used as a moderator.
Difference between Heavy and Ordinary Water