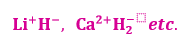Test Questions on Test # 2
on Chapter # 2
…… Hydrogen
Short Answer Questions
Q1.Complete and balance the following equations:
(i) H+ + H2O →
(ii) NaH + H2O →
(iii) CaH2 + HCl →
(iv) Mg3P2 + H2O →
(v) LiH + BH3 →
(vi) CaH2 + H2O →
(vi) CH3OH + H2O →
(viii) Mg3N2 + H2O →
(ix) H‒ + H2O →
(x) NaH + AlH3 →
Q2 What
happens when?
(i) Action
of steam on coal
(ii) Action
of steam on methanol
(iii) Action
of Hydride ion on acid
(iv) Action
of nascent hydrogen on oxygen
(v) Action
of soda lye on carbon monoxide
(vi) Aluminum
phosphide is hydrolyzed
(vii) Action
of steam on marsh gas
(viii) Action
of steam on water gas.
(ix) Action
of water on sodium hydride
(x) Action
of water on calcium
Q3. Briefly
explain:
(i) Hydrogen
exhibits +1 and -1 oxidation states in its compounds.
(ii) Why
atomic hydrogen is more reactive than the ordinary molecular hydrogen?
(iii)Write
down Electronic configuration of simplest ions of hydrogen. Show their
reactions with water.
(iv) Give
the reaction of H+ and H‒ ion with water.
(v) D2O
is heavier than H2O.
(vi) Hydrogen
is misfit in group VIIA of the periodic table. Explain
(vii) Write
equation of reaction when steam is passed over red hot coke and natural gas.
(viii) What
are isotopes? Explain the different isotopes of hydrogen.
(ix) Hydrogen
can be placed with the Alkali metals of group IA and halogens. OR give the similarities and dissimilarities of hydrogen with the elements
of group IA and VIIA
Q4. What
are ionic hydrides? Explain their preparation and properties.
Q5. What
are covalent hydrides? Explain their preparation and properties.
Q6. What
are complex Hydrides? Give their mechanism of formation and properties.
Descriptive Answer
Questions
Q7. What is water gas? How it is prepared from coal and natural gas? Give two methods of separation of hydrogen from water gas.
OR
Describe industrial methods of preparation of H2 gas from
coke, natural gas methanol, and ammonia.
Q8. What
are binary compounds and hydrides?
Define six kinds of hydrides. Explain its first three types.
Q9. Differentiate between atomic and
nascent hydrogen with their method of preparation. Give action of Nascent
Hydrogen on phosphorus, oxygen, tungsten oxide, arsenic, FeCl3 and
sodium.
Solution of Test Questions on Test # 2 on Chapter # 2 ……
Hydrogen
Q2. Briefly
explain:
(i) Hydrogen
exhibits +1 and -1 oxidation states in its compounds.
Because
having only one electron gives the option of filling the first energy level or
emptying it by gaining or losing one electron, giving oxidation states of -1
and +1 respectively.
Hydrogen
atom with electronic configuration 1s1 has only one electron. In order to
complete its outer shell it needs one electron. So when hydrogen is combined
with electropositive metals, it gets an electron forming H- ions showing
-1 oxidation state e.g. such as in NaH, CaH2 etc.
When
hydrogen combines with a more electronegative non-metallic element, it can
donate its single electron turning into H+ showing +1 oxidation
state (But H+ does not independently exists)
(ii)Why atomic
hydrogen is more reactive than the ordinary molecular hydrogen?
Atomic
hydrogen is much more reactive than molecular hydrogen. The high reactivity of
atomic hydrogen is explained by considering that it is in atomic form and
possesses extra energy.
In
molecular hydrogen, where H–H bond is strongly bonded by covalent bond, a high
bond dissociation energy of 104 kcal/mole is required to break the hydrogen
molecule into atomic hydrogen before they react. Such a high energy is not
supplied ordinarily. That is why their reactions are slow.
On the contrary, the atoms of atomic hydrogen are ready at
once for the reaction to proceed and there is no need to supply 104 kcal/mole
to the reaction. That is why atomic hydrogen reacts readily and vigorously.
(iii) Write
down Electronic configuration of simplest ions of hydrogen. Show their
reactions with water.
OR
Give the reaction of H+ and H‒ ion
with water.
Hydrogen forms two types of ions:
H+ with electronic configuration 1s0
H- with electronic configuration 1s2.
Both these ions are unstable and react
with water
H+
+ H2O → H3O+
H‒ + H2O → OH‒
+ H2
(v)D2O
is heavier than H2O.
Deuterium reacts with oxygen to form Deuterium Oxide (D2O)
which is commonly called Heavy Water or deuteride due to being 1.1 times
heavier than ordinary water. Its molecular mass is also higher than ordinary
water.
(vi) Hydrogen
is misfit in group VIIA of the periodic table. Explain
Hydrogen is misfit in group VIIA of the periodic table on
account of following differences:
Hydrogen has
one electron in valence shell which consists of s-orbital (1s1)
while group VIIA elements have 7 electrons in valence shell which consists of s
and p orbitals (ns2 np5).
H2 is colourless gas, but
halogens are coloured gases.
H2 collects at cathode while
halogens at anode during electrolysis.
H2 forms H+ ion;
halogens do not form cation.
Oxide of hydrogen (H2O) is
neutral but oxides of halogens (Cl2O7) are acidic.
Electron affinity of hydrogen is much
less than halogens.
H2 is reducing agent, but
halogens are oxidizing agent.
H–
ion is unstable while X– ions are stable. H– ion is
incapable of existence in water because it reacts with H2O to
liberate H2 gas immediately but X– ions do not react with
H2O.
The maximum covalency of hydrogen is only
1 while that of halogens is 7.
Hydrogen cannot be central atom but
halogens are frequently act as a central atom.
(vii) Write
equation of reaction when steam is passed over red hot coke and natural gas.
Water gas or synthesis gas’ or 'syngas’ is prepared by passing steam over red hot coke at about 1000°C or by passing a mixture of
steam and natural gas (containing 80% methane) over nickel at 900°C.
The process of producing 'syngas' from coal is called ‘coal gasification’.
(ix) Hydrogen
can be placed with the Alkali metals of group IA and halogens. OR give the similarities and dissimilarities of hydrogen with
the elements of group IA and VIIA.
It resembles alkali metals with respect to electronic
configuration, electropositive character, valency, oxidation
state, combination with electronegative elements, reducing
behaviour, cation formation, hydration of cations in water
and liberation at cathode.
Comparison
of Hydrogen with Halogens
It resembles halogens with respect to electronic
configuration, ionization energy, electronegative character, oxidation state,
diatomic nature (atomicity) and liberation at anode.
(viii) What are
isotopes? Explain the different isotopes of hydrogen.
Definition
“Isotopes are atoms of the same element
having same atomic number but different mass numbers (atomic masses). In other words isotopes are different forms
of atoms of an element which have same number of protons (and also electrons)
but different number of neutrons in their respective nuclei”.
Different isotopes of an element have same chemical
properties due to their identical electronic configuration (i.e. same number of
electrons in the shells) but they have different physical properties because of
their different atomic masses.
Isotopic Forms of Hydrogen
Hydrogen exists in three isotopic forms
1.
Protium.
2.
Deuterium.
3. Tritium.
Summary of Characteristics of Isotopes of Hydrogen
Q2. Complete and balance the following equations:
Q3. What happens when?
Q4. What are ionic hydrides? Explain their
preparation and properties.
Answer
Definition of ionic hydrides
the hydrides in
which hydrogen is present as hydride (H–) ion in combination with
highly electropositive s-block elements, alkali metals (group IA) and alkaline
earth metals (group IIA) except Be and Mg are called ionic hydrides.
Reason for
calling Ionic Hydrides as true hydrides
Ionic
hydrides are called as true hydrides due to presence of anionic hydrogen
ion i.e. hydride ion (H-). In fact, binary compounds are
named by their anion and in ionic hydrides hydrogen exist as its anionic
hydride form. e.g.
Reason for calling Ionic Hydrides as saline or salt like hydrides
Ionic hydrides are also known as saline
or salt like hydrides because of their ionic nature of bond and their salt
like properties.
They have following general
formula:
Preparation of Ionic Hydrides by
direct heating metal in a current of H2 gas
They respective metal hydrides are formed when the
s-block elements are heated at different temperatures in a current of hydrogen
gas. Except Be and Mg, all alkaline earth metals form ionic hydrides.
Physical Properties
Chemical Properties
1.Reducing action on oxides and Chlorides
2. Action of protonic solvent to liberate H2 gas along with base or salt
1.Reducing
action on oxides and Chlorides
They are powerful reducing agent in
metallurgical process. Due to their ability to release hydrogen, ionic hydrides
are powerful reducing agent and hence reduces many substances like oxides and
chlorides.
2. Action
of protonic solvent to liberate H2 gas
along with base or salt
They
react with protonic solvents (i.e. solvents giving H+ ions) such as
water, acids, alcohols, ammonia to evolve hydrogen gas along with base or salt.
Q5.What are
covalent hydrides? Explain their preparation and properties.
Answer
Definition
These are the hydrides of
non-metallic elements of the p-block elements of group IIIA, IVA, VA, VIA and
VIIA in which their atoms are linked to hydrogen through shared pair of
electrons or covalent bond having hydrogen in its formal oxidation state of +1.
hydrogen forms molecular compounds with most of the p-block elements. For convenience hydrogen compounds of
non-metals have also been considered as hydrides.
Examples
Group IIIA hydrides .…..BH3
or B2H6 (diborane)
Group IVA hydrides .….. CH4,
C2H4, C2H2, C6H6,
SiH4 (silane),
Group VA hydrides.….. NH3,
PH3 (P2H6; diphosphine), AsH3
(arsine), SbH3 (stibine)
Group VIA hydrides .….. H2O,
H2S, H2Se, H2Te
Group VIIA hydrides .….. HF, HCl, HBr, HI
Preparation
1.By
Direct Union of Free Elements with Hydrogen at high temperature
2. By
Hydrolysis of Binary Compound of Metals (metal borides, carbides, nitrides,
phosphides)
Properties
Types of Covalent Hydrides According to Polarity
Nature of covalent hydride is determined by the difference of E.N.
value between H and non-metallic element.
(a) Non-Polar covalent hydrides are formed with non-metal of identical electronegativity and are insoluble in water and non-electrolyte
e.g. group IVA hydrides like CH4, SiH4
(b) Polar covalent hydrides are formed with non-metal of different electronegativity and are soluble in water and electrolyte in aqueous solution
e.g. hydrides of group VA,
VIA, VIIA like H–Cl, HF, NH3.
Types of Covalent Hydrides According to number of electrons
and Bonds present
Depending upon the relative number of
electrons and bonds present in their Lewis structure, molecular hydrides have
been classified as
1. electron deficient hydrides (Hydrides
of group III (BH3, AlH3)
2. electron exact hydrides or electron
precise hydrides (Hydrides of group IV (CH4, SiH4, SnH4,
PbH4)
3. electron rich hydrides (Hydrides
of group V, VI and VIIA)
Q6. What
are complex Hydrides? Give their mechanism of formation and properties.
Definition
Some of the elements of IIIA group like B and Al (and Ga) are
combined covalently with four hydrogen atoms instead of three to form complex
anion (of the type [XH4] –) which unite with alkali metal
to form complex hydrides.
e.g.
Lithium borohydride ---------- LiBH4
Sodium borohydride ----------- NaBH4
Lithium aluminohydride ------ LiAlH4
Sodium aluminohydride ------ NaAlH4
General Formula
Complex hydrides have general formula ABH4 OR AXH4;
where,
A is monovalent metallic ion of group IA e.g. Li+, Na+.
B or X is trivalent
metallic ion of group IIIA e.g.
B3+, Al3+.
Preparation by Combination of
Electron-rich alkali metal hydrides with electron-deficient hydrides of group
IIIA in presence of ether solution
They are prepared by combination of
electron deficient hydrides of group IIIA with hydrides of alkali metals.
Mechanism of Formation
Properties
9. Action
of water to liberate H2 gas
They are soluble in water, giving alkali
metal ions (as Na+, Li+) and complex anions (as AlH4–),
which reacts with water to liberate H2 gas along with respective
hydroxides of both metals. Lithium borohydride reacts with water even in cold
evolving hydrogen gas along with lithium metaborate.
ABH4 + 4H2O
→ AOH + B(OH)3 +4H2
NaAlH4 + 4H2O
→ NaOH + Al(OH)3 + 4H2
LiAlH4 + 4H2O → LiOH +
Al(OH)3 +4H2
NaBH4 + 4H2O →
NaOH + B(OH)3 +4H2