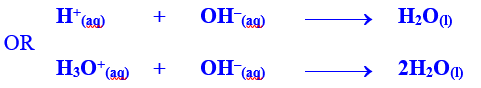Chapter # 2
Acids, Bases and Salts
For Class X
(According to New Syllabus 2022)
10.1 Acids and Bases
Introduction
Acids, bases and salts are three distinct classes in which almost all the organic and inorganic compounds are classified. A famous Muslim Chemist Jabir Bin Hayan prepared nitric acid (HNO3), hydrochloric acid (HCl) and sulphuric acid (H2SO4). In 1787, Lavoisier named binary compounds of oxygen such as CO2 and SO2 as acids which on dissolution in water gave acidic solutions. Later on in 1815, Sir Humphrey Davy discovered that there are certain acids which are without oxygen, e.g. HCl. Davy proved the presence of hydrogen as the main constituent of all acids. It was also discovered that all water soluble metallic oxides turn red litmus blue, which is a characteristics of bases.
We all have a little concentration of hydrochloric acid in our stomach, which helps to break down the food. Sometimes, the amount of stomach acid becomes too much, which causes ‘acidity’. This uncomfortable feeling is easily treated by taking an alkaline medicine. The alkali neutralizes the acid, producing a harmless chemical called a salt.
How many food items are sour? In taste lemons, oranges, grapefruits and other citrus fruits have sour taste. This sour taste is due to an acid called citric acid in them.
Bases (alkalis), on the other hand have bitter taste and are soapy to touch. When you wash your hands with soap or brush your teeth with toothpaste, you are using a base.
Whereas, salts are formed by the reaction of an acid and a base. Table salt, baking soda, washing soda etc. are some of the salts which are commonly used.
Some Household Acids and Bases/ Acid Source and Base Source
Citric acid is found in Citrus fruits i.e., lemon, oranges
Butyric acid is found in Rancid butter
Sour milk contain lactic acid
Malic acid is found in Apples
Tartaric acid is found in Tamarind, grapes, apples
Stings of ants and bees contain formic acid
Uric acid is found in Urine
Stearic acid is found in Fats
Ammonia a common base is used for cleaning purposes
Sodium hydroxide commonly known as lye a common alkali, is used as drain cleaner and for cleaning ovens
Milk of magnesia (suspension of magnesium hydroxide, Mg(OH)2 in water) and aluminium hydroxide; AL(OH)3 which are bases are used as an antacid to relieve discomfort caused excessive acidity due to excess of hydrochloric acid in the stomach.
The word acid is derived from a Latin word “acidus’ meaning sour as acids have sour taste. The first acid known to man was acetic acid, i.e., in the form of vinegar. They turn blue litmus paper red. They change the colour of acid-base indicator. Their aqueous solution are electrolyte and conduct electric current. Strong acids are very corrosive (destroy fabric and cause burns on skin).
A base is any metal oxide or hydroxide that reacts with an acid to produce salt and water. They turn red litmus paper blue. They change the colour of acid-base indicator. Their aqueous solution are electrolyte and conduct electric current. A water soluble base is called an alkali. In aqueous solution, they produce hydroxide (OH−) ions. All alkalis are bases but all bases are not alkalis.
Mineral Acids
Following acids are called mineral acids.
Hydrochloric acid (HCI)
Sulphuric acid (H2SO4 )
Nitric acid (HNO3)
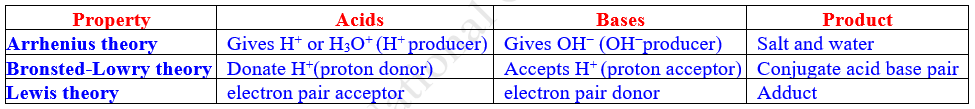
10.2 Different concepts
of Acids and Bases
2. Lowry - Bronsted concept (Proton-transfer Concept)
3. Lewis concept (Electronic Concept)
10.2.1 The Arrhenius Theory
Introduction
Svante Arrhenius, a Swedish
scientist in 1787 first defined acids and bases on their ionic dissociation
in water in his theory of ionization. He explained that aqueous solution
of acids and bases conduct electric current by producing ions in aqueous
solution. According to Arrhenius theory, acids are substances that dissociates
in water to give hydrogen ions H+(aq) and bases are
substances that produce hydroxyl ions OH–(aq).
Arrhenius Definition of Acid
An acid is a substance that dissociates in aqueous solution to yield
hydrogen ions or protons (H+).
The general ionization of an acid HY(aq) can be represented by the following equations.
For Example
Substance like Halogen acids
(HX; HCl, HBr, HI, HF), hydrocyanic acid (HCN), nitric acid (HNO3),
sulphuric acid (H2SO4), phosphoric acid (H3PO4),
acetic acid (CH3COOH), etc. are typical acids as they ionize in aqueous
solution to produce H+ ions
(but H3BO3 is not an Arrhenius acid). [Thus all acids contain
hydrogen but not all hydrogen- containing substances are acids].
Arrhenius Definition of Base
A base is a substance that gives
hydroxide (OH–) ions in aqueous solution. (Some
bases are the compounds that react with water to remove a hydrogen ion leaving
hydroxide ions in the solution.
For Example
The general ionization of a base MOH(aq) (BOH) can be represented by the following equations.
The substance like Sodium hydroxide (NaOH), Potassium
hydroxide (KOH), Ammonium hydroxide (NH4OH), calcium hydroxide Ca(OH)2 etc. are considered as bases as they ionize in aqueous
solution to furnish OH– ion in water.
Examples of Some Important Acids and bases
Limitations of Arrhenius Concept/
Drawbacks of Arrhenius Concept
1. This concept is restricted to aqueous
solution. This concept is applicable only in aqueous medium and does not explain
nature of acids and bases in non-aqueous medium.
2. It failed to account for the basicity or acidity of substances that do not contain H+ (e.g. CO2) or OH– ions (e.g. NH3)
According to this concept, acids and bases are only those compounds which contain hydrogen (H+) and hydroxide (OH–) ions, respectively. It can’t explain the acidic nature of compounds like CO2, and basic nature of NH3, etc.
Although this concept has limited scope yet, it led to the
development of more general theories of acid-base behaviour.
10.2.3 Bronsted- Lowry Theory [Proton-donor &
acceptor Theory/Proton transfer theory]
In 1923, the Danish chemist Bronsted and the English chemist Lowry
independently presented their theories of acids and bases on the basis of proton-transfer.
According to this concept:
An acid is a substance (molecule or ion) that can donate a proton
(H+) to another substance. A base is a substance that can accept a
proton (H+) from another substance. For example, HCl acts as an acid
while NH3 acts as a base:
Lowry-Bronsted Definition of Acids
A Bronsted-Lowry Acid is defined as a substance or specie (molecule or ion) that can donate one or more protons (hydrogen ions or H+ ions) to another substance. In short, acids are proton donor.
In other words, an acid is a substance that gives oxonium ion or hydroxonium ion (H3O+) in aqueous solution by donating H+ ions.
For Example
HCl in water is proton donor and thus
acts as a Bronsted-Lowry acid. [i.e. donates proton to water to form H3O+
and Cl– which are conjugate acid and conjugate base respectively.
The products of this reaction are themselves acid and base which are called
conjugate acid and conjugate base respectively]
Bronsted- Lowry Definition of Base
A Bronsted-Lowry base is defined as a
substance or specie (molecule or ion) that tends to accept a proton (H+
ions) from another substance. In short, bases are proton acceptor. In other
words, a base is a substance that combines or adds with oxonium ion or
hydroxonium ion (H3O+) or conjugate acid accepting or
removing a proton forming water i.e. accepts proton from oxonium ion or
conjugate acids to form water or OH– ion. The products of this
reaction are themselves acid and base that are called conjugate acid and
conjugate base respectively].
Drawbacks of Bronsted-Lowry Concept
It has been observed that there are certain substances which behave
as acids though they do not have the ability to donate a proton, e.g. SO3.
Similarly, CaO behaves as a base but it cannot accept a proton. These observations
prove the limitations of Bronsted-Lowry concept of acids and bases.
1. According
to this concept, proton donor and proton acceptor must co-exist. But there are
many reactions in which this does not happen.
2. It also could
not explain the behaviour of those acids and bases which do not contain
hydrogen at all i.e. it does not explain acidic behaviour of aprotic acids like SO2, SO3, CO2, AlCl3,
SiCl4 etc.
Summary of Bronsted-Lowry Concept
Acid is a proton donor and base is a proton acceptor.
Acid
---------------------------------which
gives H+ in any solvent
Base
--------------------------------- which accepts H+ in any solvent
To find out conjugate base of any acid
----- remove one H+ from acid
To find out conjugate acid of any base
----- add one H+ in base
Conjugate Acid-Base Pair
Acids and bases occur as conjugate acid-base pair (the word conjugate means “joined together or tie together as a pair”) which are defined as an acid and a base that differ only in the presence or absence of a proton or pair of acid and base that are related to each other by loss or gain of a proton.
Every acid has a conjugate base which is the negatively charged or neutral specie formed by the removal or release of a proton from the acid. A conjugate base is a species that results when an acid loses a proton.
Every base is associated with a conjugate acid which is the positively charged ion produced by the acceptance or addition of a proton by a base. The species that results when a base accepts a proton from an acid is called the conjugate acid.
For example
Consider the dissociation reaction of a
general monoprotic acid HA in water in which HA dissolves in water in a
reversible manner by donating a proton to water. Therefore, HA is the
Bronsted-Lowry acid and H2O is the base. It is seen that the
products of acid-base reaction (H3O+ and A−)
are themselves acids and bases which are called conjugate acid and conjugate
base respectively. In each acid-base reaction an acid reacts with a base to give a
conjugate base and a conjugate acid.
A conjugate
acid is a specie formed by accepting a proton by a base.
A conjugate
base is a specie formed by donating a proton by an acid.
Thus, conjugate acid-base pair differs from
one another only by a single proton.
Conjugate Acid-base Pairs of Common
Species
Amphoteric or Amphiprotic Substance
(that can accept as well as lose H+)
According to Bronsted-Lowry concept, an acid and a base always work
together to transfer a proton. That means, a substance can act as an acid
(proton donor) only when another substance simultaneously behaves as a base
(proton acceptor). Hence, a substance can act as an acid as well as a base,
depending upon the nature of the other substance. For example, H2O
acts as a base when it reacts with HCl and as an acid when it reacts with
ammonia such as:
Such a substance that can behave as an acid, as well as, a base is
called amphoteric. Water is amphiprotic
solvent (that can accept as well as lose H+)
Important Note
All Arrhenius acids are Bronsted acid but it is not so for bases i.e. All Arrhenius acids are Bronsted-Lowry acids, but except OH− other Bronsted-Lowry bases are not Arrhenius bases
Some Arrhenius hydroxides or bases such as NaOH are not
Bronsted-Lowry bases. It is because these hydroxides are not proton acceptors
whereas, the OH− ion produced in a solution is the Bronsted-Lowry
base because it is the specie that can accept a proton.
10.2.4 Lewis Concept of Acids and Bases (Electronic Concept)
Significance
The Arrhenius and Bronsted-Lowry concepts of acids and bases are limited to substances which contain protons. AlCl3, BF3, BCl3, ZnCl2, FeCl3 are considered as acids although they do not have hydrogen. In 1923, an American Chemist G.N. Lewis proposed a more general and broader electronic concept of acids and bases focusing on electron transfer instead of proton transfer. Lewis concept can be applicable to non-aqueous solutions or solutions lacking hydrogen ions and reactions that do not involve hydrogen ions at all.
The Lewis definition is much more useful than the others because it can be applied to all species and reaction. The other definitions are only useful for species and reactions involving H+.
According to
this concept:
An acid is a substance (molecule or ion) which can
accept a pair of electrons, while a base is a substance (molecule or ion) which
can donate a pair of electrons.
Lewis Definition of Acid
An acid is any species (molecule or
ion) which can accept a (lone) pair of electrons during a reaction i.e. Lewis Acids are an electron pair acceptor. They are
electrophile and have vacant orbitals. H+ is a Lewis acid.
characteristics
of a Lewis acid
1. They are also called Electrophiles (meaning electron loving)].
2. A Lewis acid must have a vacant orbital into which it can
accept the electron pair.
3. Lewis
acids include not only H+ (protons) or H3O+
(oxonium ion) but also other cations and neutral molecules having vacant valence orbitals like AlCl3, AlBr3,
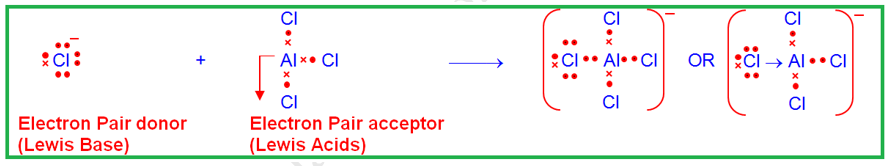
BF3, BCl3, ZnCl2, FeCl3 etc.
4. A Lewis acid-Lewis base reaction gives Lewis adduct. Lewis acid-base
reactions in general do not have to involve H+
Example between boron trifluoride and ammonia
a reaction
between ammonia and boron trifluoride takes place by forming a coordinate
covalent bond between ammonia and boron trifluoride by donating an electron
pair of ammonia and accepting that electron pair by boron trifluoride.
Example between Proton and Ammonia
to give Ammonium ion
The cations (proton itself or metal ions)
act as Lewis acids. For example, a reaction between H+ and NH3,
where H+ acts as an acid and ammonia as a base. H+
ion acts as Lewis acid because it is short of two electrons for completion of
its duplet and thus capable of accepting a lone pair of electrons from N of
ammonia which is a Lewis base making co-ordinate covalent bond.
The product
of any Lewis acid-base reaction is a single specie, called an adduct. So,
a neutralization reaction according to Lewis concept is donation and
acceptance of an electron pair to form a coordinate covalent bond in an adduct.
In other words, an acid-base neutralization reaction involves donation of an
electron pair from a base to an acid forming a coordinate covalent bond.
Acids are
electron pair acceptors while bases are electron pair donors. Thus, it is
evident that any substance which has an unshared pair of electrons can act as a
Lewis base while a substance which has an empty orbital that can
accommodate a pair of electrons acts as Lewis acid.
Example or Types of Lewis
Acids
Compounds having less than eight electron
in their valence shell or positively charged ions that can accept an electron
pair can act as Lewis acids.
1. Having incomplete octet e.g. BF3, BCl3, B(OH)3,
AlCl3 etc.
2. Having multiple bonds between atoms of different E.N e.g. CO2, SO2,
SO3 etc.
3. Having vacant d-orbitals e.g. SF4, SF6, SnCl2,
SnCl4 etc.
4. All cations Li+, Ag+, Al3+, Mg2+ etc. The cations (proton itself or metal ions) act as Lewis acids.
False cations (which cannot act as Lewis acid) e.g. Na+, K+, Ca2+, NH4+, PH4+, H3O+
(i) Molecules in which the central atom has incomplete octet. For example, in BF3, AICI3, FeCl3, the central atoms have only six electrons around them, therefore, these can accept an electron pair.
(ii) Simple cations can act as Lewis acids. All
cations act as Lewis acids since they are deficient in electrons. However,
cations such as Na+, K+, Ca2+ ions, etc., have
a very little tendency to accept electrons. While the cations like H+,
Ag+ ions, etc., have a greater electron accepting tendency
therefore, act as Lewis acids.
Lewis
Definition of Base
A base is any species (molecule or
ion) which can donate a (lone) pair of electrons during a reaction i.e. Lewis bases are electron pair donor. They are nucleophile and have lone pair
of electrons.
characteristics
of a Lewis Base
1. They are also called
Nucleophiles (meaning nucleus loving)].
2. Lewis
bases include not only OH– ions but also all anions (F–,
Cl– etc.) and neutral molecules having lone pair of electrons (NH3,
C2H5OH etc.).
3. Ammonia is base in all
three concepts.
Example between Ammonia and Boron
trifluoride to give ammonia-boron trifluoride adduct
ammonia is
a Lewis base as it donates its an
electron pair to boron of boron trifluoride lacking a pair of electrons to
complete its octet acting as Lewis acid to form
co-ordinate covalent bond.
Example between Chloride ion and
aluminium chloride to give complex anion as adduct
Examples or Types of Lewis Bases
Compounds having lone pair of electron in
the valence shell or negatively charged ions that can donate an electron pair
can behave as Lewis bases.
1. Neutral molecules having lone pair (unshared pair) of electrons e.g.
NH3, H2O, R-NH2, R2NH, ROR etc.
2. All anions e.g. O2−, SO42−,
CO32−, Cl−, Br−, I−, CH3COO−
etc.
1. Neutral molecules having lone pair
of electrons
Neutral species having at least one lone pair of electrons can act
as a Lewis base. For example, ammonia, amines, alcohols etc. act as Lewis bases
because they can donate their lone pair of electrons:
2. All
Anions or Negatively charged species
All anions or negatively charged species can donate electron pair
so they are Lewis base. For example, chloride, cyanide, hydroxide ions, etc.,
act as Lewis bases:
All the Lewis bases are Bronsted bases but all the Lewis acids are not Bronsted acids
It may be noted that all Bronsted bases are also Lewis bases but all Bronsted acids are not Lewis acids.
According to Bronsted concept, a base is a substance which can accept a proton, while according to Lewis concept, a base is a substance which can donate a pair of electrons. Lewis bases generally contain one or more lone pair of electrons and therefore, they can also accept a proton (Bronsted base). Thus, all Lewis bases are also Bronsted bases.
On the other hand, Bronsted acids are those which can give a
proton. For example, HCI, H2SO4 are not capable of
accepting a pair of electrons. Hence, all Bronsted acids are not Lewis
acids.
Basicity of Acids OR Protocity of Acids
Definition of Basicity of the Acid
Different acids have different number of acidic hydrogen
(or protons) per molecule and yield different number of oxonium (H3O+)
ion in aqueous solution. Acids that contain two or more acidic hydrogen per
molecule are called Poly-basic or Poly-protic acids
(e.g. H2SO4, H2CO3, H2C2O4,
H3PO4 etc.)
“The number
of ionizable or replaceable acidic hydrogen atoms (or protons; H+
ions) present in a molecule of an acid is called Basicity of the acid.”
Types of Acids on the basis of Basicity
Monobasic Acids Or
Monoprotic Acids
1. The acids that contain only one replaceable acidic hydrogen atom (or proton; H+ ion) per molecule are called Monobasic or Monoprotic acids. [When one mole of such acids is dissolved in water, they produce 1 mole of hydrated proton, H+ i.e. oxonium ion (H3O+)].
2. They
can neutralize only one mole of OH– ions.
They dissociate in one steps forming only one type of anionic
specie.
Examples
of Monoprotic Acids
HCl, HBr, HI, HF, HNO3, HCOOH, CH3COOH, H3BO3 etc.
Dibasic Acid OR Diprotic Acids
1. The acids that contain two replaceable acidic hydrogen atoms (or protons; H+ ions) per molecule are called Dibasic or Diprotic acids. They are also called Poly-protic acids.
2. They
can neutralize two moles of OH– ions.
They dissociate in two steps forming two types of anionic
species.
Examples
of Diprotic Acids
H2SO4,
H2CO3, H2S, H2C2O4,
H3PO3 etc.
Tribasic Acids OR Triprotic Acids
1. The acids that contain three replaceable acidic hydrogen atoms (or protons; H+ ions) per molecule are called Tribasic or Triprotic acids. They are also called Poly-protic acids.
2. They
can neutralize three moles of OH– ions.
They dissociate in three steps forming three types of anionic
species.
Examples
of Triprotic Acids
H3PO4, citric
acid (C6H8O7/ CH2COOH-C(OH)(COOH)-CH2COOH),
Arsenic acid (H3AsO4)
Polybasic Acids OR Polyprotic Acids
1. Acids that contain two
or more acidic hydrogen per molecule are called Poly-basic or Poly-protic acids.
2. They dissociate
in two or three steps forming two or three types of anionic
species.
Examples
of Polyprotic Acids
H2SO4, H2CO3, H2C2O4, H3PO4
Acidity of Bases Or Hydroxility of Bases
Definition
Different bases have different number of hydroxide (OH–) ions per molecule and thus yield different number of OH– ion in aqueous solution. Bases that contain two or more OH– ions per molecule are called Poly-acid Bases.
“The number of ionizable or replaceable hydroxide (OH–) ions present in a molecule of a base is called Acidity of the base.”
Types of Bases on the basis of
Acidity
Monoacid base
They have one replaceable hydroxide (OH–) ion and produce 1 mole of hydroxide (OH–) ions per mole of base in aqueous solution. When one mole of such bases are dissolved in water, they produce 1 mole of OH–
They can neutralize only one mole of H+ ions. They dissociate in one step.
Examples of Monoacid base
NaOH, KOH, NH4OH etc.
Diacid base
They have two replaceable hydroxide (OH–) ions and produce 2 moles of hydroxide (OH–) ions per mole of base in aqueous solution.
They can neutralize only two moles of H+ ions. They dissociate in two steps.
Examples of Diacid base
Ca(OH)2, Ba(OH)2, Zn(OH)2, Mg(OH)2 etc.
Triacid base
They have three replaceable hydroxide (OH–) ions and produce 3 moles of hydroxide (OH–) ions per mole of base in aqueous solution.
They can neutralize only three moles of H+ ions. They dissociate in three steps.
Examples of Triacid base
Fe(OH)3, Al(OH)3, Cr(OH)3 etc.
Polyacid base
Bases that contain two or more OH– ions per molecule are called Poly-acid Bases.
They can neutralize only two or three moles of H+ ions. They dissociate in two or three steps.
Examples of Polyacid base
Ca(OH)2, Ba(OH)2, Zn(OH)2, Mg(OH)2, Fe(OH)3, Al(OH)3, Cr(OH)3 etc.
Basicity of Lewis Bases
The basicities of Lewis bases decrease
sharply as the number of unpaired pairs of electrons (lone pairs) increases.
10.2.5 Neutralization
1st Definition (Arrhenius Definition)
The type of double displacement (decomposition) reaction in which
equivalent quantities of two reactants acid and base react to form salt and
water is called Neutralization. It is the reverse of process of hydrolysis.
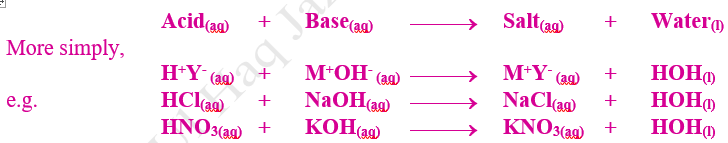
2nd Definition (Arrhenius Definition)
Since neutralization reaction always
involves the reaction between a H+ ion of acid and a OH– ion of base, therefore, water is the
resultant product. During neutralization, cation of base and anion of acid
remains in solution and do not react. These ions are called spectator ions.

Thus neutralization may also be defined
as:
the process of the mutual chemical
combination of hydrogen ions or protons (H+) of an acid and
hydroxide ions (OH–) of a base to form neutral water molecule is
termed as neutralization.
Thus neutralization reactions can be
denoted by a single net –ionic equation:
Definition
of neutralization in Lewis
Concept
The product of any Lewis acid-base reaction is a single specie, called an adduct. So, a neutralization reaction according to Lewis concept is
donation and
acceptance of an electron pair to form a coordinate covalent bond in an adduct. In other words, an acid-base
neutralization reaction involves donation of an electron pair from a base to
an acid forming a coordinate covalent bond.
Heat of Neutralization
Neutralization is an exothermic reaction releasing heat called heat of neutralization.
the amount of heat evolved during neutralization in which one mole of water is formed (when one gram equivalent of an acid neutralizes completely one gram equivalent of a base in a dilute solution) is called heat of neutralization.
In other words,
Heat of neutralization is the amount of heat evolved when 1 mole of
H+ ions of an acid reacts with 1 mole of OH- ions of a
base to form one mole of water. In fact, heat of neutralization is
the heat of formation of water from H+ and OH- ions.

In case of weak acid or base or both,
heat of neutralization < 13.7 kcal/mol and the difference is enthalpy of
ionization of weak species except in case of HF when heat of neutralization
> 13.7 due to hydration of F- ions.
The amount of heat of neutralization for any strong acid against any strong base is approximately same i.e. ‒13700 calories/mol or ‒57.3 kJ/mol.
The heat of neutralization for weak acid
against strong base or strong acid against weak base (i.e. in case where either
acid or base is not completely ionized and the neutralization reaction may not
go to completion) may be less than 13700 cal. e.g. when strong acid HCl reacts
with a weak base Ca(OH)2, heat of neutralization is 24700 or 12350
cal/mol.
Types of Neutralization
Neutralization
is of two types
(a) Complete
Neutralization
(b) Partial
Neutralization
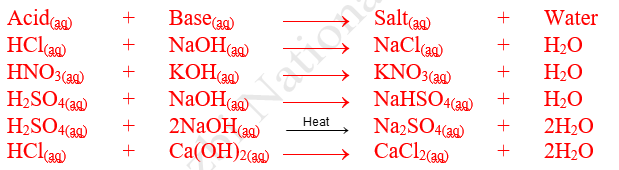
(a) Complete Neutralization
A neutralization in which all H+ ions of the acid are neutralized by OH– ions of base or vice versa is known as complete neutralization.
Salts obtained by complete neutralization do not have replaceable hydrogen atoms or hydroxide ions and are normal (neutral)
e.g. NaCl, NaBr, NaI, NaNO3, KCl, Na2SO4, K2SO4 etc.
(b) Partial Neutralization
A neutralization in which all H+
ions are not neutralized by OH– ions of the base or vice versa (i.e.
all OH- ions of a base are not neutralized by the H+ ions
of the acid) is known as Partial Neutralization. Salts obtained by partial
neutralization have either replaceable hydrogen ions or hydroxide ions and are
either acidic or basic.
Salts formed by the
partial neutralization of an acid by base containing replaceable hydrogen ion
are acidic. They further react with bases to form normal salts.
e.g. NaHSO4, KHSO4, KHCO3,
Na2HPO4, NaH2PO4, Na2HPO4,
K2HPO4, NaH2PO4, KH2PO4
etc.

Salts formed by the
partial neutralization of a base by an acid containing replaceable hydroxyl ion
are basic. They further react with acids to form normal salts.
e.g. Ca(OH)NO3, Mg(OH)NO3, Zn(OH)NO3,
Pb(OH)NO3, Pb(OH)Cl, Zn(OH)Cl etc.
General Physical properties
of Acids and Bases

10.3 Physical Properties of Acids

10.4 Uses of Acids
1.
Hydrochloric acid is used
for cleaning metals, tanning and in printing industries.
2. Nitric
acid is used in
manufacturing of fertilizer (ammonium nitrate), explosives, paints, drugs and
etching designs on copper plates.
3. Sulphuric
acid is used to
manufacture fertilizers, ammonium sulphate, calcium superphosphate, explosives,
paints, dyes, drugs. It is also used as an electrolyte in lead storage
batteries.
4. Acetic acid is used for flavouring food and food
preservation. It is also used to cure the sting of wasps.
5. Benzoic acid is used for food preservation.
Stomach acidity
Stomach
secretes chemicals in a regular way to digest food. These chemicals mainly
consist of hydrochloric acid along with other salts. Although, hydrochloric
acid is highly corrosive, but stomach is protected from its effects because it
is lined with cells that produce a base. The base neutralizes stomach acid. The
important function of this acid is to break down chemical bonds of foods in the
digestion process. Thus, big molecules of food are converted into small ones.
It also kills the harmful bacteria of certain foods and drinks.
However,
sometimes stomach produces too much acid. It causes stomach acidity also called
hyperacidity. Symptoms of this disease are feeling burning sensation throughout
the gastro intestinal track. These feelings sometimes extend towards the chest,
that is called heart burning.
The best
prevention from hyperacidity is:
i)Avoiding over-eating and
staying away from fatty acids and spicy foods.
ii) Simple and regular eating, remaining in
an upright position for about 45 minutes after taking a meal.
iii) Keeping the head elevated while sleeping.
Process of
Etching in Art and Industry
The process
of etching on glass is carried out by using a wax stencil. Stencil is placed on
areas of glass or mirror that are to be saved from acid. The glass or mirror is
dipped into hydrofluoric acid. The acid dissolves the exposed part of the glass
thus etching it. This process has been very dangerous because the acid would
damage the skin and tissue of artist’s body. Although, it is dangerous to deal
with acid, yet etching done with acid is very attractive as compared to using
other chemicals.
10.7 Chemical Properties of Acids
1) Neutralization
Reaction with Bases/alkalis to
generate salt and water
Acids react
with bases (oxides and hydroxides of metal and ammonium hydroxide) to form
salts and water. This process is called neutralization.
2) Neutralization
Reaction with Metallic Oxides to generate salt
and water
Acids react
with basic metallic oxides and amphoteric metal oxides to form salts and water.
This process is also known as neutralization.
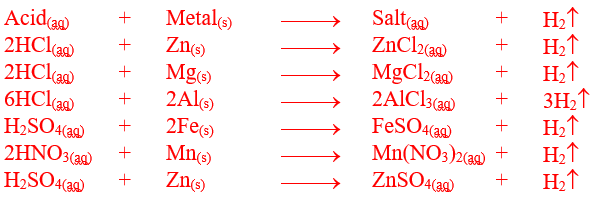
3) Displacement
Reaction with Metals in Dilute Form to generate salt and hydrogen gas
Highly
electropositive metals lying above H in reactivity series reacts with dilute
acids to displace H from it in the form of hydrogen gas along with respective
salt. Acids react explosively with metals like sodium, potassium and calcium.
However, dilute acids (HCl, H2SO4) react moderately with
reactive metals like Mg, Zn, Fe and Al to form their respective salts with the
evolution of hydrogen gas.
4)Double
decomposition Reaction With Metallic Carbonates & Bicarbonates to generate salt, water
and CO2
Acids react
with carbonates and bicarbonates to form corresponding salts with the evolution
of carbon dioxide gas.
5)Double
decomposition Reaction With Metallic Sulphites and Bisulphites to generate salt, water and SO2
Acids react
with sulphites and bisulphites to form corresponding salts liberation of
sulphur dioxide gas.

6) Double
decomposition Reaction With Metallic Sulphides to generate salt
& hydrogen sulphide gas
Acids react
with metal sulphides to form respective salt and liberate hydrogen sulphide
gas.