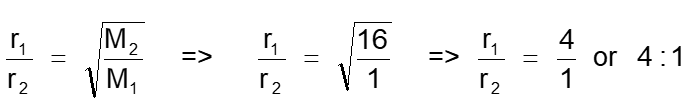MCQs Set 2 with Explanatory Answers
1. The mass of one mole of electrons is:
(b) 0.55 mg
(c) 0.184 mg
(d) 1.673 mg
|
|
Reason
mass of 1 electron = 9.1×10−31 kg =
9.1 × 10−25 mg
1 mole of electron = 6.02 × 1023 electrons
Mass
of 1 mole of electrons = mass of 1 electron × number
of one mole of electron
= (9.1 × 10−25) × (6.02×1023)
= 0.54782mg ≈ 0.55mg
The mass of one mole of electrons = 0.55 mg
2. The number
of moles of CO2 which contain 8.0 g of oxygen:
(b)0.50
(c)1.0
(d)1.50
Reason
Mass of 1 mole or Molar
mass of CO2 = 12 x 16 x 2 = 44 g/mol
Mass of oxygen in 32 g
of CO2 = 16 x 2 = 32 g
32 g of oxygen is
contained in 1 mole of CO2
1 g of oxygen is
contained in 1/32 mole of CO2
8 g of oxygen is
contained in = 1/32 x 8 = 0.25 mole of CO2
3. The largest number of molecules are present in:
(a)3.6 g of water
(b)4.8 g of C2H5OH
(c)2.8 g of CO
(d)5.4 g of N2O5
Reason
Hence 3.6 g
water having greatest number of moles out of given options will contain largest
number of molecules.
4. Which of the following
formula represents an acid anhydride?
Reason
Oxides
are called anhydride. Anhydrides are of two types:
1. Basic anhydrides or basic oxides
which are oxides of metals like Na2O, MgO, CaO etc.
2. Acid anhydrides or acidic oxides
which are oxides of non-metals like SO2, SO3, CO2,
NO2, Cl2O etc.
Out
of given option, CaO is basic anhydride, H2O is a neutral oxide
(other examples includes NO, N2O, CO), H2SO4
is an oxyacid itself and SO3 is an acid anhydride. SO3
dissolves in water to form H2SO4 (an oxyacid) .
5. If an element M forms an
oxide with formula MO, which of the following is a correct formula?
Reason
In MO,
oxidation number of M is +2. In MS and MCl2, oxidation number of M
is also +2.
6. The formula of KAl(SO4)2
represents a total of:
KAl(SO4)2
= 1K + 2Al + 2S + (4 x 2)O= 12 atoms
7. How many neutrons are
contained in the nucleus of an element with Z=27 and A= 59:
No of
neutrons = A – Z = 59 – 27 = 32
8. How many gram of NaOH
are needed to make 1000 g of a 5% solution?
9. What is
the ratio of the weight of water formed to the weight of Hydrogen used in the formation
of water?
10. Which one
of the following compounds does not
have the empirical formula CH2O?
Explanation
11. Equal
masses of methane and hydrogen are mixed in an empty container. At 25°C. The
fraction of total pressure exerted by
hydrogen will be?
|
|
Let x g of both gases be mixed.
12. A small spacecraft of capacity 10 m3 is connected to another of capacity 30 m3. Before connection, the pressure inside the smaller craft is 50 kPa and that inside the larger is 100 kPa.
If all measurements are made at the same temperature, what is the pressure in the combined arrangement after connection?(a) 150 kPa
(b) 75 kPa
(c) 87.5 kPa
(d) 100 kPa
Reason
13. The
densities of two gases are in the ratio of 1: 16. The ratio of their rates of
diffusion is
Reason
14. The molecules of a gas A travel four times faster than the
molecules of gas B at same temperature. The ratio of molecular weights (MA / MB) is
Reason
15. A mixture of H2 and O2 in 2:1 volume
is allowed to diffuse through a porous partition what is the composition of gas
coming out initially
Reason
16. Which one of the following properties has the same value for
hydrogen (H2) and for deuterium (D2) when compared at the
same pressure and temperature?
17. The molecules of a gas A travel 8 times faster than the
molecules of gas B at same temperature. The ratio of molecular weights (MA / MB) is