Hybrid Molecular Structure of Methane
In methane i.e. CH4, carbon is bonded to four hydrogen atoms, thus carbon gets sp3-hybridized. Each H – C bond is sigma bond which is formed due to s-sp3 overlapping. There are four sigma bonds in CH4. Thus CH4 has tetrahedral geometry. Each bond angle in CH4 is 109.28° (109.5°). The bond length between C–H is 1.09
Brief
Description of Shape of Methane
Hybrid Molecular Structure of Ethene (Ethylene)
In ethene i.e. C2H4,
each carbon is bonded to three other atoms (i.e. 2 H and 1 C atom) thus each
carbon uses sp2 hybrid orbitals plus unhybrid pz orbital
to make its bonds.
One sp2-hybrid
orbital of each carbon atom overlaps linearly with other carbon to form a CH
sigma bond. The remaining two sp2-hybrid orbitals of each carbon
overlaps with 1s orbitals of hydrogen atoms to form four C-H sigma bonds. Now
unhybridized pz orbitals of each carbon overlap on parallel axis to
form a pi bond between carbon to carbon.
There are five sigma bonds in
C2H4, 4 in between C and H and one in between C and C,
while one Pi bond in between C and C. Thus C2H4 has
trigonal geometry. All bond angles (H-C-H and H-C-C) are 120°. Each C-H bond
length is 1.09°A and each C=C bond length is 1.34°A (shorter than C-C bond
length in CH3-CH3 which is 1.54°A).
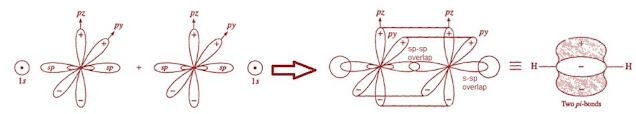
Hybrid Molecular Structure of Ethyne (Acetylene)
In ethyne (acetylene), each
carbon is bonded to two atoms (i.e. one H atom and one C atom) thus each carbon
uses sp hybrid orbitals plus two unhybrid py and pz
orbitals to make its bonds.
One sp-hybrid orbital of each
carbon atom overlaps linearly with sp orbital of other carbon to form a C-C
sigma bond. The remaining sp-hybrid orbital of each carbon overlap with 1s
orbital of Hydrogen atoms to form two C-H sigma bond. Two unhybrid
atomic orbitals (2py and 2pz) of each carbon overlap on
parallel axis to form two pi bonds between C to C. All bond angles (H-C-C) is
180○. Each C-H bond length is 1.09○A and each C-C bond
length is 1.2○A (which is shorter than C-C and C=C bond lengths).
Hybrid Molecular Structure of Ethane