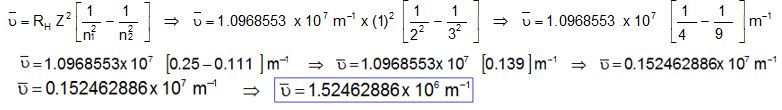Q1. Calculate the wave
number and wavelength of Balmer series of hydrogen spectrum in which electron
jumps from orbit 3 to orbit 2.
Solution
Calculation of Wave Number
Here;
RH = 1.0968553 x 107 m-1
n1 = 2
n2 = 3
Z = 1
Calculation of Wave Length
Q2. What is the wavelength and wave number of radiation that is
emitted when a hydrogen atom undergoes a transition from orbit 3
to orbit 1.
Solution
Calculation of Wave Number
RH = 1.0968553 x 107 m-1
n1 = 1
n2 = 3
Q3. Calculate the ratio of radius of second and
third orbit of hydrogen atom. (Bohr’s radius = 0.529ºA)
Solution
Q4. If the radius of first Bohr orbit is ‘x’ then calculate the radius of the third orbit of hydrogen.
Solution
Q1.Calculate the energy of the electron in the ground state and first excited state of the hydrogen atom
Solution
Part
(A)
Z for H = 1
n for ground state
= 1