Learn the Real Secret Behind Molecular Stability 💡 | Learn Chemistry by Inam Jazbi
Resonance Explained in 5 Minutes ⚡ | Mesomerism Made Easy | Class XI–XII Chemistry
Understand Resonance (Mesomerism) easily with step-by-step explanations, rules, and examples from Class XI & XII Chemistry. Learn how electron delocalization stabilizes molecules — perfect for board exams and entrance tests.
What is Resonance / Mesomerism?
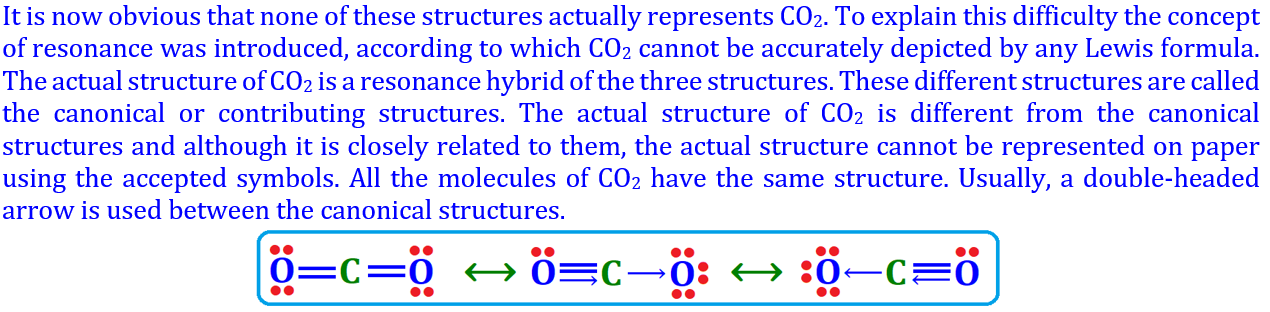
Resonance or Mesomerism is the phenomenon where a molecule cannot be represented by a single Lewis structure but by two or more contributing structures that differ only in the position of electrons, not atoms.
The real structure is a hybrid of all possible structures, called the resonance hybrid, and is more stable than any single contributing structure.
⚛️ Example: Benzene (C₆H₆)
Benzene has two possible resonance structures:
The actual structure is a hybrid, with delocalized π-electrons spread over the entire ring — explaining its equal bond lengths and stability.
🔹 Important Points to Remember:
🧩 Examples of Resonance Compounds
| Compound | Resonance Effect |
|---|---|
| Benzene (C₆H₆) | Delocalization of π-electrons |
| Carbonate ion (CO₃²⁻) | Three equivalent structures |
| Nitrate ion (NO₃⁻) | Equal N–O bond lengths |
| Amide group (–CONH₂) | Partial double bond character |
✨ Quick Trick to Identify Resonance:
If you see multiple bonds next to lone pairs or π-bonds, it’s a clue that resonance might occur!
🧭 Summary Chart
| Term | Definition | Key Feature |
|---|---|---|
| Resonance / Mesomerism | Delocalization of electrons | Stabilizes molecule |
| Resonance Hybrid | Average of all forms | Real structure |
| Arrow (↔) | Shows resonance | Not actual equilibrium |
💥 Why Students Love This Concept
-
Helps in predicting stability of molecules
-
Explains bond length and energy differences
-
Very important in Organic Chemistry and exam MCQs
-
Makes reaction mechanisms easier to understand