Welcome to Learn Chemistry by Inam Jazbi!
In this post, we’ll discuss the Exceptions to the Octet Rule, also known as the failure or limitations of the octet rule — a very important topic from Class XI Chemistry (Chapter: Chemical Bonding). The octet rule states that atoms tend to gain, lose, or share electrons to achieve eight electrons in their outermost shell, just like noble gases. However, many molecules do not follow this rule. Examples include incomplete octet (BF₃, BeCl₂), expanded octet (PCl₅, SF₆), and odd-electron molecules (NO, NO₂). These exceptions show the limitations of the octet theory and highlight the need for the modern orbital concept.
Learn the Exceptions to the Octet Rule (Failure or Limitations) with examples like BF₃, PCl₅, and NO. Complete Class XI Chemistry notes for 2025 exams.
Exceptions to Octet Rule
Failure or Limitations of Octet Rule
Octet Rule Examples and Explanation
Class XI Chemistry Notes 2025
Chemical Bonding Exceptions
#OctetRule #ChemistryNotes #ChemicalBonding #XIClassChemistry #LearnChemistry #InamJazbi #Chemistry2025 #Class11
🧪 Exceptions to Octet Rule (Failure or Limitations of Octet Rule)
Introduction
⚛️ 1. Incomplete Octet
Some elements form stable compounds even when the central atom has less than eight electrons.
These atoms are usually from Groups 2 and 13 of the periodic table.
| Example | Central Atom | Valence Electrons Around Atom |
|---|---|---|
| BeCl₂ | Be | 4 electrons |
| BF₃ | B | 6 electrons |
| AlCl₃ | Al | 6 electrons |
🧠 Reason: Small and highly electropositive atoms like Be, B, and Al have low electronegativity and can’t attract enough electrons to complete the octet.
🌟 2. Expanded Octet
Some elements can have more than eight electrons in their valence shell.
This occurs in period 3 and below, where d-orbitals are available for bonding.
| Example | Central Atom | Valence Electrons |
|---|---|---|
| PCl₅ | P | 10 |
| SF₆ | S | 12 |
| XeF₄ | Xe | 8+4 = 12 |
🧠 Reason: These atoms use empty d-orbitals to accommodate extra electrons beyond the octet.
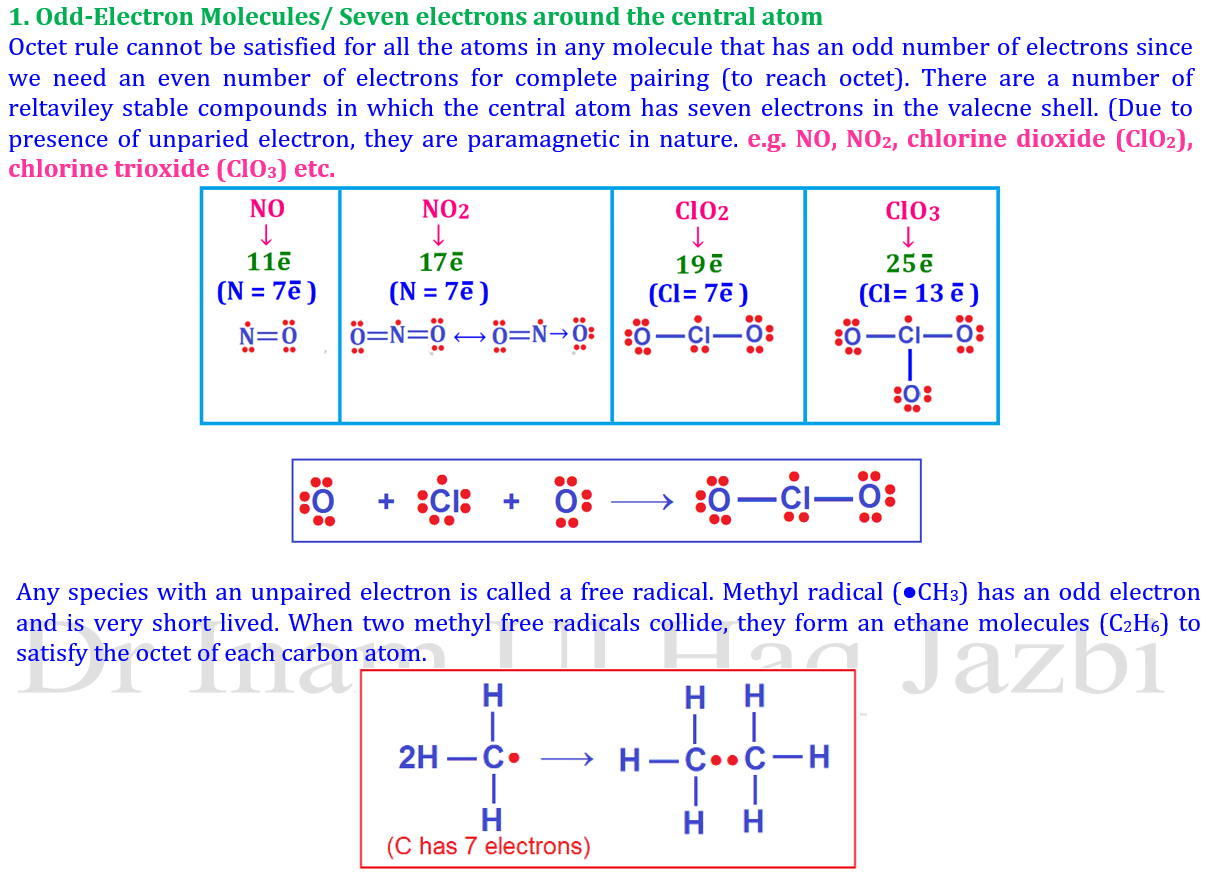
⚡ 3. Odd-Electron Molecules
Some stable molecules have an odd number of total electrons, so it’s impossible for all atoms to have an octet.
| Example | Molecule Type | Unpaired Electron |
|---|---|---|
| NO | Free radical | 1 |
| NO₂ | Free radical | 1 |
| ClO₂ | Free radical | 1 |
🧠 Reason: These molecules contain unpaired electrons, making them paramagnetic and reactive.
🧩 4. Ionic Compounds with Transition Metals
Many transition metals and heavy elements do not follow the octet rule because they have variable oxidation states and incomplete d-orbitals.
Examples: Fe²⁺, Cu²⁺, Ni²⁺, etc.
🔍 Limitations of Octet Rule
-
It does not explain the stability of molecules with an odd number of electrons.
-
It fails for molecules where atoms have less or more than eight electrons.
-
It cannot explain the shape and magnetic properties of molecules.
-
It does not apply to transition elements.
🧾 Conclusion
The Octet Rule is useful for understanding simple molecules, but it has clear limitations. Many compounds show incomplete, expanded, or odd-electron configurations, which can only be explained using modern bonding theories like the Orbital Concept and Valence Bond Theory.