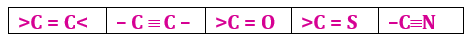Reasons of large number of
Organic Compounds/Some Features of Organic Compounds / Diversity and
Magnitude of Organic Compounds
There are a total of 118 elements known today. Although the earth’s crust contains only 0.027% carbon, millions of organic compounds are known. The number of organic compounds is more than ten million. This number is far more than the number of compounds of all the remaining elements taken together. Carbon forms such a large number of organic compounds due to its following some special behaviour and peculiar properties
1. Peculiar nature of
Carbon
(i) Tetravalency
(ii) Catenation
(a unique property of carbon)
(iii) Moderate electropositivity
2. Electronegativity and strength of bonds
3. Multiple Bonds forming tendency
4. Isomerism
5. Covalent nature or
non-ionic character
6. Similarity in
behaviour (homologous series)
7. Complexity in
structure
8. Solubility only in
non-polar solvents
9. Low melting and
boiling points
10. High volatility and
highly flammable characters
11. Non-conductance of electricity
12. Slow rates of
organic reactions with low yield
13. Polymerization
1. Tetravalency
Carbon is
tetravalent and show tetravalency. It can unite with four monovalent atoms at a
time.
The
tetravalent nature and tetrahedral structure of carbon was introduced by Liebel and Van’t
Hoff
The tetravalency of carbon was proved by Kekule in 1857.
2. Catenation (a unique property of carbon)
One of the remarkable property of carbon atom is its unique capacity to form bonds with other carbon atoms. This property of forming bonds with atoms of the same element is called catenation.
The property of carbon atoms to bond or link
itself to other carbon atoms forming long chains, branched chains, rings or
compounds with chains and rings together is called Catenation. It is property of self-linking of carbon atoms through covalent
bonds to form long straight or branched chains and rings of different sizes.
The self-linking property of forming bonds with atoms of the same element is called catenation. It is the capacity of atoms to build long chain or huge rings by linking with other similar atoms.
[Any number of carbon atoms can unite with each other through single, double or triple covalent bonds to form stable chains and rings of any size and length].
The ability of carbon atoms to join with another via covalent bonds to create long Straight or branched chains or rings of carbon atoms is the primary cause for the formation of vast number of organic compound.
basic Criteria or conditions for Catenation
Two basic conditions for an element to exhibit catenation are:
1. Element should have
valency two or greater than two.
2. An element’s bonds with its own atoms should be stronger than the element’s bonds with other atoms, particularly oxygen.
Both silicon and carbon have similar electronic configurations but
carbon shows more catenation whereas silicon exhibits very less. It is mainly
due to the reason that C-C bonds are much stronger (355 kJ mol−1)
than Si-Si (200 kJ mol−1) bonds. On the other hand, Si - O bonds are
much stronger (452 kJ mol−1) than C-O bonds (351 kJ mol−1).
Hence, silicon occurs in the form of silica and silicates in nature.
Reason of Catenation
Carbon shows maximum catenation in the periodic table and this property is primarily due to its small size, unique electronic configuration and maximum bond energy or greater strength of carbon- carbon bonds as compared to other atoms for catenation (C > Si > S > P > O > F).
Reason of Catenation of Carbon
The tendency of carbon for catenation is due to:
(i) Unique electronic
arrangements.
(ii) Tendency for forming strong covalent bonds capable of holding greater no. of carbon atoms.
For example, C–C bond is very strong (335 kJ mol-1) in comparison to Si–Si bond (220 kJ mol-1) or Ge–Ge bond (167 kJ mol-1). As a result, carbon atoms can link with each other to form either linear chains of various lengths or branched chains and even rings of different sizes as shown below:
(iii) Isomerism
Another reason for the abundance of organic compounds is the
phenomenon of isomerism. Carbon compounds show phenomenon of isomerism
by virtue of which a single molecular formula may represent two or more
compounds. The compounds are said to be isomers if they have the same
molecular formula but different arrangement of atoms in their molecules
or different structural formulae. Isomerism also adds to the possible
number of structures. Number of isomers increases with the increase in number
of carbon atoms in the given molecular formula.
e.g.
Pentane with molecular formula C5H12 can be represented by three different structures. Thus, C5H12 has three isomers, as shown below:
C2H6O is the molecular formula of two isomers
(iv) Multiple Bonds forming tendency
In order to satisfy its tetravalency, carbon can make multiple bonds (i.e., double and triple bonds) with other carbon atoms and also with other atoms like O, S and N due to its small size. This further adds to the possible number of structures.
For example, two carbons in ethane are linked by a single covalent bond, by a double covalent bond in ethylene and a triple covalent bond in acetylene.
(v) Electronegativity and Strength of covalent bonds of carbon
The electronegativity of carbon (2.5) is close to a number of other elements like H (2.1), N (3.0), P (2.1), Cl (3.0), O (3.5). Due to its very small size and moderate electronegativity, carbon can form very strong covalent bonds with other carbon atoms, hydrogen, oxygen, nitrogen and halogens. This enables it to form a large number of different compounds.