Welcome to Learn Chemistry by Inam Jazbi 👨🔬. In this blog, we’ll break down Electronic Configuration and its Rules in the simplest way possible — perfect for FSC (Intermediate) students and MDCAT aspirants.
Electronic
Configuration and its Rules
Definition Electronic Configuration
The distribution of electrons in the available orbitals in the order of increasing energy is called Electronic Configuration.
The electronic configuration describes the exact position of electron in extra-nuclear region i.e. available orbitals. Thus electronic configuration is the filling up of orbitals in the sequence of increasing energy.
🌟⚛️ Some Unexpected Electronic Configuration🌟⚛
⚡The elements of group VIB i.e. Cr, Mo and W have expected valence shell electronic configuration (n-1)d⁴, ns² but in practice their configuration is (n-1)d⁵, ns1.
e.g.
✨Chromium has 24 electrons and its expected electronic configuration is 1s², 2s² 2p⁶, 3s² 3p6, 3d⁴, 4s² but in reality the configuration is 1s², 2s² 2p⁶, 3s² 3p⁶, 3d⁵, 4s¹.
⚡The elements of group IB i.e. Cu, Ag and Au have expected valence shell electronic configuration (n-1)d⁹, ns² but in practice their configuration is (n-1)d¹⁰, ns¹.
e.g.
✨copper with Z = 29 has the electronic configuration of 1s², 2s² 2p⁶, 3s² 3p⁶, 3d¹⁰, 4s¹ instead of 1s², 2s² 2p⁶, 3s² 3p⁶, 3d⁹, 4s¹
🌟⚛Factors responsible for the extra stability of half-filled and complete filled sub-shells 🌟⚛
⚛(i) Symmetrical
Electronic Distribution
The symmetrical electronic distribution leads to stability. Thus the electronic configuration with all the orbitals of the same subshell are either fully filled or exactly half filled are more stable due to symmetrical distribution of electrons.
⚛(ii) Exchange energy (Cause of Extra Stability of Half-filled &
Completely Filled Orbitals)
The electrons with parallel spins present in the degenerate orbitals (orbitals of same subshell having equal energy) tend to exchange their position. The energy released during this exchange is called exchange energy. The number exchanges that can take place is maximum when the degenerate orbitals are exactly half-filled or fully filled. As a result, the exchange energy is maximum and so it the stability.
The exchange energy is represented as
It is both in backward and forward direction which is counted as 1.
If more number of exchanges are possible, more exchange energy is released. More number of exchanges are possible only in the case of half-filled and fully filled configurations. More are the number of electrons with identical spin, more are the number of ways of exchanging with other electrons, more will be the exchange energy released which lead to greater stability.
✨For example
If the electronic configuration of chromium was [Ar] 3d⁴ 4s², then electrons could be exchanged in only six ways releasing less exchange energy. (i.e. In case of 3d⁴ 4s² configuration in Cr; the electron no.1 can exchange its position with electrons nos. 2, 3 & 4 i.e., in 3 ways. The electron no. 2 can exchange 2 ways with electrons 3 & 4 (with electron 1 has already been considered). The electron no.3 can exchange only in 1 way (as exchanges with electrons 1 & 2 has already been calculated). Hence there are 3+2+1=6 ways of exchange are possible in 3d4 arrangement).
From spectroscopy, the electronic configuration of chromium is [Ar] 3d⁵ 4s1. in 3d⁵ configuration, the total possible ways of exchange is 4+3+2+1=10. The 3d orbital is half filled and there are ten possible exchanges. Hence, exchange energy for the half filled configuration is more This increases the stability of half filled 3d orbitals. Therefore, 3d⁵ configuration is more stable than 3d4 configuration
The reason for this is, Cr with 3d⁵ configuration is half filled and it will be more stable. Chromium has [Ar] 3d⁵ 4s1 and not [Ar] 3d4 4s² due to the symmetrical distribution and greater exchange energies of d electrons.
🌟⚛ Methods of Writing Electronic Configuration🌟⚛
🎯1. Orbital
Method
In this method, the electrons present in respective orbitals are denoted.
Cl (17) = 1s², 2s² 2p⁶, 3s² 3p⁵ OR [Ne] 3s² 3p⁵
🎯2. Shell
method
In this method, the number of electrons in each shell is continuously written
Cl (17) = 1s², 2s² 2p⁶, 3s² 3p5
K², L8, M7
2, 8, 7
🎯3. Box
Method
In this method, each orbital is denoted by a box and electrons are represented by half-headed (↿⇂) or full-headed arrows. An orbital can occupy a maximum of two electrons
🎯4. Core
Noble gas EC Method
In this method, the electrons present in the valence shells are shown while electrons in core shells are shown by writing nearest noble gas in square bracket.
Cl (17) = 1s², 2s² 2p⁶, 3s² 3p⁵ OR [Ne] 3s² 3p⁵
🔥🌟Exceptions in Electronic Configurations🔥🌟
🌟⚛Rules for Electronic Configuration
Pauli’s Exclusion Principle
Introduction and Statement
It is an empirical rule but agrees fully with experimental observations (and facts) but has no mathematical explanation. It was put forward by Wolfgang Pauli in 1925. It is used to assign the values of four quantum numbers to an electron of an atom
No two electrons in an atom (in the same orbital) can have
the same set of four quantum numbers. Thus an orbital can contain a maximum of
two electrons with opposite spins.
In other words,
the set of four quantum numbers associated with an electron acts as a unique “address” for that electron in an atom, and no two electrons can have the same address.
The two electrons in the same orbital will have identical values of n, l and m but due to opposite electron spin (clockwise and anticlockwise), they must have different values of spin quantum number (+ ½ and - ½).
Theoretical Proof
An electron is an spinning negative charge and an spinning charge is magnetic, therefore, an spinning electron can be considered to act like a tiny magnet. Two electrons with opposite spins in an orbital will behave as two tiny magnets with opposite poles towards each other and thus attract each other.
Electrons with same spin repel each other and occupy different orbitals and if they have to occupy the same orbital, they must have opposite spins. This is in accordance with Pauli’s exclusion principle.
Examples
In He (Z = 2), there are two electrons in K-shell. It can be seen that these two electrons have same values for n, l and m but due to opposite electron spins, they have different value of spin quantum number.
Applications or Significance
1. According to Pauli’s exclusion principle, an orbital can contain maximum of two electrons with opposite spins, as spin quantum number (ms) has only two possible opposite values.
2. Thus electrons occupying the same orbital have opposite spin i.e. spin gets opposite when electrons pair up after occupying singly.
3. Two electrons in the same orbital with opposite spins are called paired electrons, symbolized as ↿⇂ . An orbital which is occupied by an electron pair is called Completely Filled Orbital.
4. An atom with x number of electrons therefore has at least x/2 orbitals, though it might have more if some of its orbitals are only half filled.
auf-bau principle/ Building up Principle/Diagonal Rule
Introduction and Statement
Auf Bau (pronounced as of bow) is a German word meaning “To construct” or “To build up” or “building up”. The Auf Bau Principle gives us a sequence in which various orbitals are filled with electrons i.e. it governs the sequence of various orbitals for feeding in electrons in them.
It is assumed that in an atom all the orbitals are vacant and
electrons fill these orbitals in the order of increasing orbital energy
starting with 1s orbital.
OR
In the ground state of an atom, the electrons tend to occupy the available orbitals in the increasing order of orbital energies starting with 1s orbital i.e. orbitals of minimum energy are filled up first with electrons and only then the orbitals of higher energy are filled. Thus electrons first enter into the lowest energy sub-shell, then enter into the next higher energy sub-shell.
Electrons are added one by one to the various orbitals in order of their increasing energy starting with the orbital of lowest energy. The increasing order of energy of various orbitals is
1s < 2s < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p
Explanation
Hypothetically, electronic configuration of atoms can build up by placing the electrons in the lowest energy orbitals until the total number of electrons added is equal to the atomic number “Z”. The notation used to indicate the number of electrons present in a given orbital is nℓx. Where,
n = 1, 2, 3 .............
ℓ = s, p, d or f
x = number of electrons actually present.
Diagrammatic Representation
The order of filling or building up of orbitals is simplified by following diagram in which the orbitals are cut diagonally; the one cut first is filled first.
Order of Filling
The sequence of increasing orbital energy is given below:
Wiswesser’s Rule OR (n + l) Rule/Madelung Rule/Klechkowsky Rule
Significance
The rule is named after William Wiswesser. This rule helps in determining the stable electronic configuration by giving sequence of energies of orbitals, hence it guides in the order of filling of orbitals by electrons.
Statement
In building up the electronic configuration of the atoms, orbitals
with lowest value of n + l fills
first; when two orbitals have the same value of n + l, then the orbital with least n value is filled first.
[Here n and l stands for the principal and azimuthal quantum No. respectively].
In multi-electron atom, subshell having higher value of (n+ ℓ) has higher energy as compared to subshell having lower value of (n+ ℓ). If (n+ ℓ) value is same then energy order of subshell is decided by the value of “n”. Greater the value of “n”, more will be the energy.
Explanation of (n + l) Rule
According to Wiswesser Rule, the order of filling of sub-shells (orbitals) is determined by increase value of n + l. An electron enters first in that orbital which has lowest n + l value. If two orbitals have the same n + l values, the electron will go into the orbital with least n value. Straight filling of electrons take place up to Argon (Atomic No. = 18) i.e. order of filling of electrons in the main energy level is same to order of orbital.
Sub-shell with lower value of n + l will have lower energy and also electrons enter first in that sub-shell. If n + l value is same for two or more sub-shells then lower n value will have lower energy.
Examples of (n + l) Rule
1. In case of potassium (Z = 19), the electron might go into 3d or 4s orbital. This will be decided by finding out n + l value for 3d and 4s orbital:
For 3d orbital; n = 3 and ℓ = 2; value of n + ℓ = 3 + 2 = 5
For 4s orbital; n = 4 and ℓ = 0; value of n + ℓ = 4 + 0 = 4
Hence 4s orbital, which has the lower value of n + l than 3d orbital, will be filled first.
2. In case of Scandium (Z = 21), electron will enter into 3d or 4p orbitals.
For 3d orbital; n = 3 and ℓ = 2; value of n + ℓ = 3 + 2 = 5
For 4p orbital; n = 4 and ℓ = 1;value of n + ℓ = 4 + 1 = 5
Hence 3d orbital fills before 4p orbital, although n + ℓ values for both are same. But 3d orbital has lower ‘n’ value.
Energy Level Sequence of Sub-shell
Increasing Energy Order of Subshell
1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p
Exceptions to the rule
in the transition metals
The valence d-subshell "borrows" one electron (in the case of palladium two electrons) from the valence s-subshell.
For example, in copper 29Cu, according to the Madelung rule, the 4s orbital (n + ℓ = 4 + 0 = 4) is occupied before the 3d orbital (n + ℓ = 3 + 2 = 5). The rule then predicts the electron configuration 1s2 2s2 2p6 3s2 3p6 3d9 4s2, abbreviated [Ar] 3d9 4s2 where [Ar] denotes the configuration of argon, the preceding noble gas. However, the measured electron configuration of the copper atom is [Ar] 3d10 4s1. By filling the 3d orbital, copper can be in a lower energy state.
Exceptions among the lanthanides
and actinides
The valence d-subshell often "borrows" one electron (in the case of thorium two electrons) from the valence f-subshell. For example, in uranium 92U, according to the Madelung rule, the 5f orbital (n + ℓ = 5 + 3 = 8) is occupied before the 6d orbital (n + ℓ = 6 + 2 = 8). The rule then predicts the electron configuration [Rn] 5f4 7s2 where [Rn] denotes the configuration of radon, the preceding noble gas. However, the measured electron configuration of the uranium atom is [Rn] 5f3 6d1 7s2.
A special exception is lawrencium 103Lr, where the 6d electron predicted by the Madelung rule is replaced by a 7p electron: the rule predicts [Rn] 5f14 6d1 7s2, but the measured configuration is [Rn] 5f14 7s2 7p1.
Hund’s Rule of Maximum Multiplicity
Introduction
The orbitals given by a particular value of ‘ℓ’ if n is same, have the same energy and such orbitals of equal energy are called Degenerate Orbitals. The filling of degenerate orbitals with electrons takes place according to Hund’s Rule of Maximum Multiplicity.
Statement
When degenerate orbitals (having equal energy) are available,
electrons occupy them singly (as they tend to be avoid each other due to repulsion)
with parallel spin. The pairing of electrons start when all degenerate orbitals
are singly occupied i.e. spin of electrons get opposite when they pair up after
occupying singly.
OR
Electrons are distributed among degenerate orbitals of a
sub-shell in such a way that maximum number of electrons occupies them singly
with same spin. When all orbitals are singly occupied only then the pairing of
electrons commences [i.e. spin gets opposite when electrons pair up after
occupying singly.]
OR
This rule is based on the fact that electrons, being of the same negative charge, repel each other and hence tend to remain far apart from each other as much as possible if they have the choice to do so. Consequently, the electrons in the orbitals of equal energy will distribute themselves in different orbitals in order to be as far apart as possible.
Explanation
Electron enters orbitals of same energy
level i.e. particular sub-shell (in 2p, 3d, 4f etc.) lonely and their spin will
be same. After entering single electron in each orbital in a particular
sub-shell, electrons enter on those half-filled orbitals and make pair but their
spin will be opposite.
Examples
(i) 2p2 means in 2p sub-shell (i.e. in three 2p orbitals) there are two electrons.
(ii) 2p5 means in 2p sub-shell (i.e. in three 2p orbitals) there are five electrons.
(iii) The electronic
configuration of carbon (Z = 6) = 1s↿⇂, 2s↿⇂ , 2px↿⇂
2py 2pz (Degenerate orbitals).
This configuration is not according to Hund’s Rule of maximum
multiplicity. Since orbitals of equal energy (px, py and
pz) called Degenerate orbitals
are available, so electrons must occupy them
separately with parallel spin. Second electron of ‘p’ will go to 2py. Thus correct electronic configuration according to Hund’s Rule is:
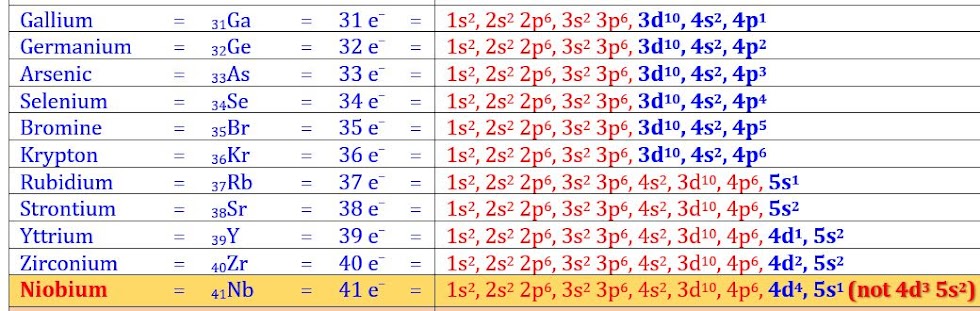
Electronic Configuration of first 50 Elements
Order of filling:
1s, 2s 2p, 3s 3p, 4s, 3d, 4p, 5s, 4d, 5p,
6s, 5d1, 4f, 5d, 6p, 7s, 6d1, 5f, 6d, 7p