Manufacture of Soda Ash By Solvay-Ammonia-Soda Process
Introduction
Sodium Carbonate (Na2CO3)
commonly called Soda Ash or Sal Soda and hydrated sodium carbonate (Na2CO3.10H2O)
generally called washing soda are commercially manufactured by Solvay’s Process or Ammonia-Soda Process which was developed in 1861 by Belgian scientist, Ernest Solvay. Sodium bicarbonate (NaHCO3)
commonly known as baking soda or cooking soda is a valuable intermediate product of Solvay’s process.
Merits of Advantages of Solvay’s Process
Solvay’s
process has following advantages:
1. It gives soda of much higher purity (99.5).
2. It is a low cost process as raw materials are very cheap and less expensive.
3.Neither wastage of materials occurs nor harmful by-products forms.
4.No pollution problems as no gaseous pollutants are formed.
5.it
is a highly
efficient process as about 97%
CO2 is used up in this process.
6.Sodium bicarbonate is obtained as a valuable intermediate.
7.Calcium chloride (CaCl2) is a
useful by-product.
8. It is a cyclic, continuous and self-contained process as raw materials like NH3 and CO2 are recovered
and recycled.
Raw Materials and their Sources
Basic Principle
The process is based on the
formation of ammonium bicarbonate by the reaction of NH3, CO2 and water which on
double decomposition with sodium chloride solution gives precipitate of sparingly soluble salt of sodium bicarbonate which is ignited to give anhydrous sodium
carbonate.
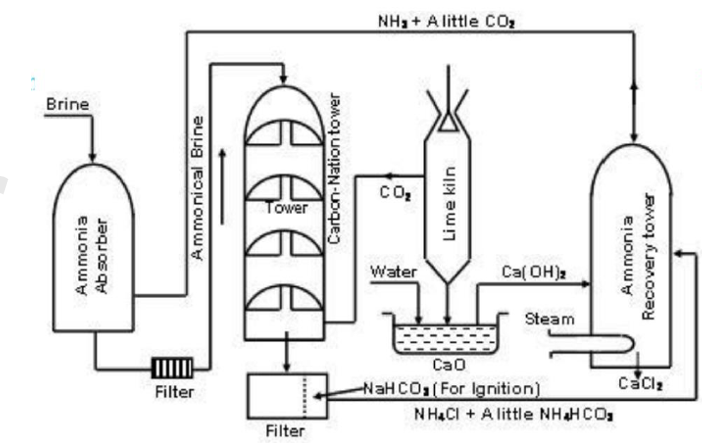
Different Steps of Process
1.Ammoniation of Brine in Ammoniating
Tower (Saturation of Brine with Ammonia).
2.Carbonation
of Ammoniated Brine in Carbonation Tower.
3.Filtration in rotary suction filter
(vacuum filtration chamber).
4.Calcination and decomposition of Sodium
Bicarbonate into soda ash and then its recrystallization
5. Ammonia
Recovery.
1.Ammoniation of Brine in
Ammoniating Tower (Saturation of Brine with Ammonia)
In the ammoniating tower (ammonia
absorber), a saturated solution of sodium chloride (about 28% by mass) called brine containing some
CO2 is thoroughly agitated with ammonia gas (coming from Ammonia Recovery Tower or
ammonia generator) until saturated ammoniated
brine is obtained.
[The tower consists of mushroom-shaped baffles at short intervals which control
the flow of brine ensuring proper saturation with ammonia passing up the
tower].
The ammonical brine so produced is allowed to stand for some time so that any impurities of
calcium, magnesium or iron salts present in brine are precipitated as carbonates and hydroxides settle down which are filtered off. The impurities settle down and clear
solution is passed through carbonating tower.
2. Carbonation of Ammoniated Brine
(a) Production of CO2
For carbonation of ammoniated
brine, CO2 is produced by heating lime stone to 1000°C in a furnace
called Lime
Kiln.
(b) Saturation of Ammonical
Brine with CO2
In Solvay or carbonation tower,
carbonation of ammonical brine is carried out on the Counter-current
process. Ammonical
brine is allowed to trickle down a carbonating (Solvay) tower (fitted with
baffle-plates) where it meets an upward current of carbon dioxide gas (coming
from a lime kiln produced by heating lime stone) introduced from the bottom of
the tower at a pressure of 1-2 atmosphere. [The baffle-plates check the flow of
ammonical brine and break up the CO2 into small bubbles to ensure
good conditions for the reaction].
Here CO2 and NH3
reacts with water to give NH4+ and HCO3–
ions which then react with Na+ and Cl– ions of brine to
precipitate less soluble sodium bicarbonate leaving ions of NH4+
and Cl– in solution. Since the overall effect of these reactions is
exothermic, the temperature of materials rises. This tends to increase the
solubility of NaHCO3 thereby inhibiting its precipitation. To
counter this adverse effect, the lower part of tower is cooled to 15°C.
3. Filtration in Rotary Suction
Filter
The intermediate product soda
bicarbonate is filtered in a vacuum filtration chamber where ammonium salts are
removed. (The thick milky suspension of NaHCO3 and NH4Cl
from the base of carbonating tower is them filtered (by means of rotary suction
filter where two products are separated). The precipitated soda bicarbonate
left on filter cloth is scrapped off after washing with a spray of cold water
and dried to free of ammonium salts. The filtrate or mother liquor containing
ammonium chloride is pumped to the ammonia recovery tower).
4. Calcination of Sodium Bicarbonate followed by recrystallization
The precipitate of NaHCO3
is ignited (in specially constructed cylindrical vessels) to give anhydrous Na2CO3
or soda ash. CO2 evolved is recycled to carbonating tower to use it
again. Washing soda (decahydrate Na2CO3.10H2O)
is produced from soda ash by re-crystallization from its hot aqueous solution.
5. Ammonia Recovery
In ammonia recovery tower, solution
of NH4Cl is made to react with quick lime; CaO (obtained from lime
kiln) or with slaked lime (obtained by reacting CaO and steam) to give NH3
which is recycled to saturation tank. CaCl2 is a by-product.
Summary
The
manufacturing of washing soda from brine involves its ammoniation in ammoniating
tower where brine is saturated with
ammonia and impurities are settled down followed by carbonation of ammoniated brine in Solvay (carbonation) tower where
brine reacts with ammonium bicarbonate to precipitate less soluble baking soda
which is thermally decomposed on calcination to yield soda ash which on
recrystallization from hot its hot aqueous solution gives washing soda.