Model Test Questions Chemistry Test # 5 for Chapter # 2 (Atomic Structure)
Short Questions Answers
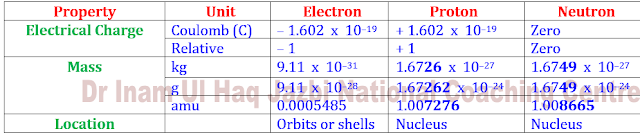
Q1. Write down the names
of sub atomic particles their masses in a.m.u with their unit charges.
OR
Write down 3 characteristics of each
fundamental particles.
Q2. Justify that
Rutherford atomic model has defects?
OR
Write down 2 defects of Rutherford’s
atomic model.
Q3. What is atomic number of an oxygen atom which has 8 electrons and 8
protons?
Q4. Find out mass number of chlorine which has 17 protons and 18 neutrons?
Q5. How many electrons, protons and neutrons are present in Co?
Q6. Do you know any element which has no
neutron in its atom?
Long Questions Answers
Q7. Describe briefly the experiments which provide clue and evidences of
electron, proton and neutron in an atom.
Q8.Discuss Rutherford'
gold metal foil experiment in the light of structure of atom.
OR
Discuss
Rutherford’s Alpha particles scattering experiment & write down postulates
of his atomic model.
Q9. Discuss discharge tube experiment for the discovery of electrons with 4
properties of cathode rays.
OR
Q10. Discuss Canal ray experiment for the discovery of protons with 4
properties of positive rays.
Model Test Questions Chemistry Test # 6 for Chapter # 2 (Atomic Structure)
Short Questions
Answers
Q1. What are Limitations
of Bohr's Atomic Model?
Q2. Differentiate
between shell and sub shell with examples?
Q3. What is maximum number of electrons that can be accommodate in's'
subshell?
Q4. How many electrons will be in L shell of an atom having atomic number 11?
Q5. In the distribution of electrons of an atom, which shell filled first and
why?
Q6. If both K and L shells of an atom are
completely filled, what is the total number of electrons are present in them?
Q7. An atom has 5 electrons in M shell than:
(a) Find out its atomic number?
(b) Write Electronic configuration of atom?
(c) Name the element of atom?
Q8. Describe wave
particle duality of electron of De Broglie Hypothesis?
Long Questions Answers
Q9. Prove that modern theory of De Broglie is related with Einstein and
Plank's equations.
Q10. Describe the schrodinger
atomic model.
Q11.Explain how Bohr's
atomic model is different from Rutherford atomic model.
OR
State
postulates of Bohr’s atomic model.
Q12. Write down electronic configuration of B, F, N, Na, P, Cl, Ca, K+,
O2-, S2-, Mg2+, Cl-.
Answer
Experiment Showing evidence of electron
Electron is the lightest particle
carrying negative charge in an atom discovered by J.J. Thomson and William
crooks.
Structure of Discharge Tube
An ordinary discharge tube consists of a glass tube fitted with two
metallic plates called electrodes connected to the high voltage battery and
vacuum pump.
Working or Observation
(Gases are bad conductor of electricity, but current can pass
through them at low pressure). When the tube is evacuated and a current of high
voltage is applied across electrodes at reduced pressure (1 to 0.001 mm Hg), a
stream of bluish light (rays) is originated and travelled in straight line from
cathode to anode and cause glow at the wall of opposite end. As these rays are
emitted from cathode, they are named as Cathode Rays.
Characteristics of
Cathode Rays
J.J. Thomson justified that these rays
were deflected towards positive plate in electric and magnetic field which
shows that these rays possess negative charge due to this negative charge,
particle was named Electron. These electrons were obtained from the gas in
discharge tube which proves that electrons are constituent of all matter.
Experiment Showing evidence of Proton
Introduction
The proton is positively charge particle
discovered by a German Physicist Goldstein in 1866. J.J. Thomson investigated
properties of proton in 1897 (who found that positive rays were composed of
positively charged particles and they were renamed as protons (meaning first).
Discovery and
Apparatus used
Goldstein used a special discharge tube with perforated cathode.
Goldstein found that in addition to cathode rays in the gas discharge tube with
perforated cathode, there were other streams of positively charge rays
travelling in opposite direction to that of the negatively charge cathode rays.
He named these rays as Positive Rays (protons). As these rays pass through the
holes of cathode they are named as Canal Rays.
Remember that canal rays are not emitted
by anode, but they are result of striking of electron with residual gas
molecules in discharge tube. Electrons ionize the gas molecules as follows.
Goldstein justify that atoms are electrically
neutral, while electrons carry negative charge. It means for each electron
there must be one equivalent positive charge to neutralize that electron. This
particle is called proton and it is a fundamental particle of all Atoms.
Experiment Showing evidence of Neutron
Discovery
An English scientist, James Chadwick in 1932 through a study of
nuclear reactions, discovered the existence of neutral particle and named it
neutron.
Chadwick found that when alpha (µ) particles
bombarded on Beryllium some penetrating radiations were given out. Chadwick
suggested that these radiations were due to material particle with mass
comparable to hydrogen atom but have no charge. These radiations (particle) are
called Neutron.
Nuclear Reaction
The nuclear reaction between Beryllium and alpha particles is
called Alpha Neutron (µ-n) reaction, during which neutron is given out in the form of very
penetrating radiations.
Introduction
On the basis of Alpha-Particle Scattering Experiment, Lord
Rutherford in 1911 not only discovered nucleus of the atom but also proposed an
Atomic Model similar to the Solar System.
Rutherford’s Gold Foil
Experiment/Alpha-Particle Scattering Experiment
Rutherford passed a beam of fast-moving
µ-particles emitting from a radioactive material source (e.g Polonium) through
a very thin gold metal foil behind
which Zinc Sulphide (ZnS)
Fluorescent Screen was placed to detect the extent of scattering of µ-particles
by gold foil.
Observation
1. Most of the α-particles passed
straight and undeflected through the sheet and produced illumination on the
zinc sulphide screen.
2. Very
few alpha (α) particles undergo small and strong deflection after passing
through gold sheet.
3. A
very few alpha (α) particles (1 out of 8000 µ-particles) bounce back and retraced their path.
Assumptions of
Rutherford’s Atomic Model (Conclusions)
According to Rutherford’s model, an atom consists of two parts namely the nucleus and extra-nuclear part. To explain this scattering of µ-particles by gold foil, Rutherford proposed the following points:
1. majority of the alpha particles passed straight line and un-deflected showing that most of the volume occupied by an atom is largely empty.
2. An
atom consists of very small positively charged central dense heavy part called
nucleus in which most of the mass of the atom is concentrated as positively
charged alpha particles show deflection in the central part. The size of the
nucleus is very small as compared to the size of its original atom.
3. The
nucleus is built of protons and neutrons which are responsible for mass of an
atom. (Since the mass of the atom is due to presence of protons and neutrons
and as these particles are residing in the nucleus, therefore, the mass of the
atom is concentrated in the nucleus. Due to proton having positive charge, the
nucleus carries the positive charge).
4. The
atom as a whole is electrically neutral, so it is concluded that the number of
protons must be equal to the number of electrons.
5. The nucleus is surrounded by large
empty space which is called extra nuclear part where probability of finding
electron is maximum. Electrons are revolving around the nucleus in the
extra-nuclear circular path called orbits or shells with very high speed.
Significance
Electron is the lightest particle
carrying negative charge in an atom discovered by J.J. Thomson and William
crooks.
Structure of Discharge Tube
An ordinary discharge tube consists of a glass tube fitted with two metallic plates called electrodes connected to the high voltage battery and vacuum pump.
Working or Observation
(Gases are bad conductor of electricity, but current can pass
through them at low pressure). When the tube is evacuated and a current of high
voltage is applied across electrodes at reduced pressure (1 to 0.001 mm Hg), a
stream of bluish light (rays) is originated and travelled in straight line from
cathode to anode and cause glow at the wall of opposite end. As these rays are
emitted from cathode, they are named as Cathode Rays.
Characteristics of
Cathode Rays
J.J. Thomson justified that these rays
were deflected towards positive plate in electric and magnetic field which
shows that these rays possess negative charge due to this negative charge,
particle was named Electron. These electrons were obtained from the gas in
discharge tube which proves that electrons are constituent of all matter.
On the basis of experimental observations, J.J. Thomson found that
cathode rays have following properties:
1. they travel in straight lines from cathode towards anode as they cast
sharp shadow of an opaque objects
placed in their path.
2. They cause a
light paddle wheel to rotate and elevate its temperature showing that they are composed of material particles.
3. they are negatively charged particles as they are bend towards the positive
plate in an electric and magnetic
field.
4. The charge to
mass i.e. e/m ratio of cathode particles is 1.76 x 108 coulomb/g i.e. same for all electrons
regardless of any gas in the tube.
5.They can
produce mechanical pressure indicating they possess kinetic energy.
6. They cause
some light sensitive materials and glass to glow or produce fluorescence.
Introduction
The proton is positively charge particle
discovered by a German Physicist Goldstein in 1866. J.J. Thomson investigated
properties of proton in 1897 (who found that positive rays were composed of
positively charged particles and they were renamed as protons (meaning first).
Discovery and Apparatus used
Goldstein used a special discharge tube with perforated cathode.
Goldstein found that in addition to cathode rays in the gas discharge tube with
perforated cathode, there were other streams of positively charge rays
travelling in opposite direction to that of the negatively charge cathode rays.
He named these rays as Positive Rays (protons). As these rays pass through the
holes of cathode they are named as Canal Rays.
Remember that canal rays are not emitted
by anode, but they are result of striking of electron with residual gas molecules
in discharge tube. Electrons ionize the gas molecules as follows.
Goldstein justify that atoms are electrically neutral, while electrons carry negative charge. It means for each electron there must be one equivalent positive charge to neutralize that electron. This particle is called proton and it is a fundamental particle of all Atoms.
Characteristics of Positive Rays
1. They travel in straight line away from anode
towards cathode.
2. They are composed
of material particles as they
produce sharp shadow of object placed in their path
3. They are composed of positively charged particles
as they bend towards the negative plate of in electric
and magnetic field. .
4.The charge to
mass i.e. e/m ratio of positive particles varies with the nature of the gas used inthe tube. The e/m ratio
of positive particle is always much smaller
than that for electron.
5.The mass of
proton is 1836 times more than electron.