List of Acid Radicals (Anions) in Salt Analysis
List
of Basic Radicals (Cations) in Salt Analysis
Analysis of dilute H2SO4/ dilute HCl Group (Acid Radicals Group I)
Experiment (Dried Test)
Original salt + dilute H2SO4 /dilute HCl + Warm if need
Confirmatory Tests of Acid
Radicals of Group I
Analysis
of Concentrated H2SO4 Group (Acid Radicals Group II)
Experiment
O. salt + conc. H2SO4 + Heat
Confirmatory
Tests of Acid Radicals of Group II
Analysis of BaCl2 / Ba(NO3)2 Group(Acid Radicals Group III)
Experiment
Soln of salt in dil. HCl + BaCl2 ----> White ppt or no ppt ------> May be SO42−/ PO43−
Note
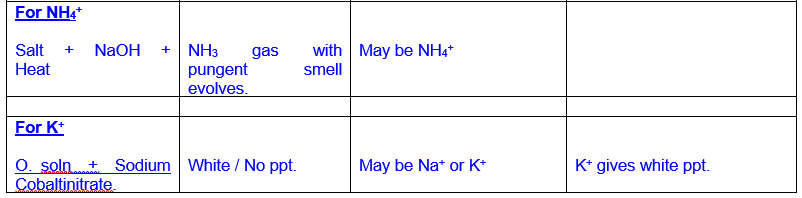
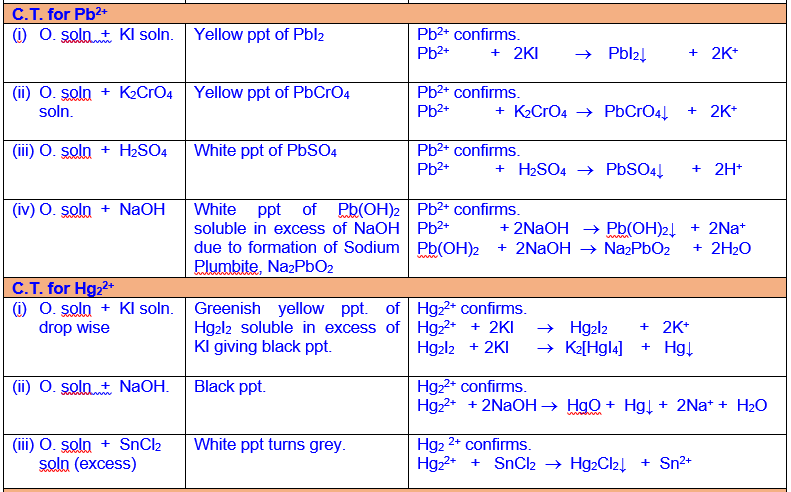
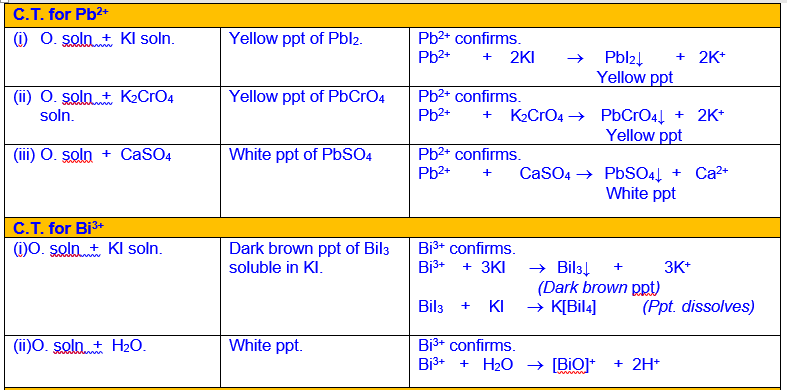
Analysis and Confirmation of Cations of Group III
Analysis
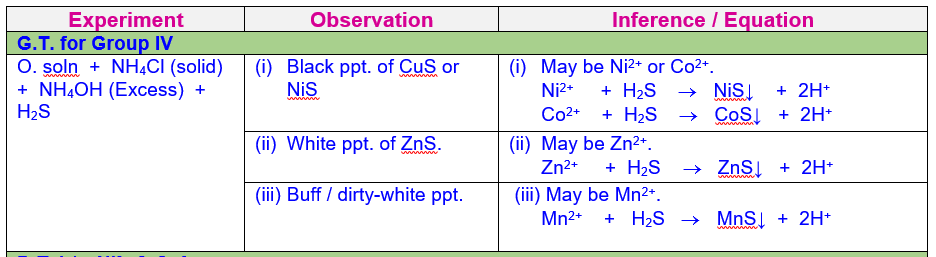
and Confirmation of Cations of Group IV
Analysis and Confirmation of Cations
of Group V
Analysis and Confirmation of Cations
of Group VI
Preliminary Examination of the Salt
Sample Paper No 1 on Salt Analysis
(Analysis of FeCl3)
Object: Identify the given salt both by dry and wet tests.
Result
Basic
radical --------- Fe3+
Acid radical ---------
Cl¯
Salt --------- FeCl3
(ferric chloride)
Sample Paper No 2 on Salt Analysis
(Analysis of NiSO4)
Object: Identify the given salt both by dry and wet tests.
Result
Basic radical ------ Ni2+
Acid radical ------- SO42−
Salt ------- NiSO4 (Nickel sulphate)
Sample Paper No 3 on Salt Analysis (Analysis of
NH4Br)
Object: Identify the given salt both by dry and wet tests.
Result
Basic radical ----- NH4+
Acid radical ----- Br−
Salt ------ NH4Br (Ammonium Bromide)
Sample Paper No 4 on Salt Analysis
(Analysis of CaCO3)
Object; Identify the given salt both by dry
and wet tests.
Sample Paper No 5 on Salt Analysis (Analysis of
Cr (NO3)2)
Object Identify the given salt both by dry and wet tests.
Result
Basic radical ------ Cr3+
Acid radical ----- NO3−
Salt ------- Cr(NO3)3
(chromium nitrate)
II. Biochemical Tests
General
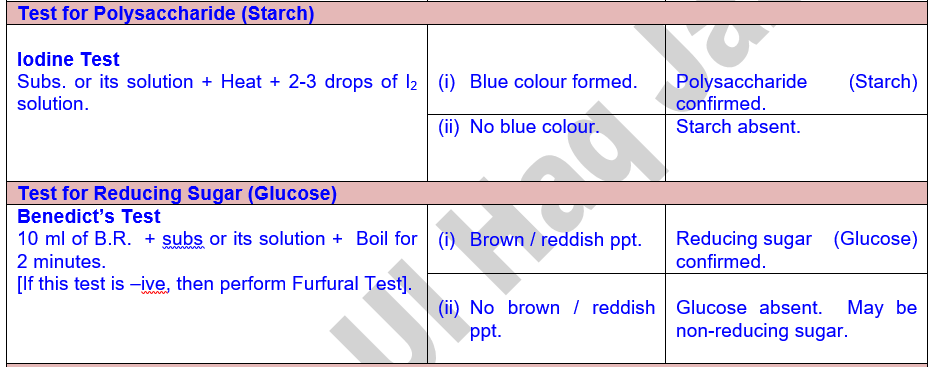
Scheme for the Detection of Carbohydrates
Tests
for Carbohydrates
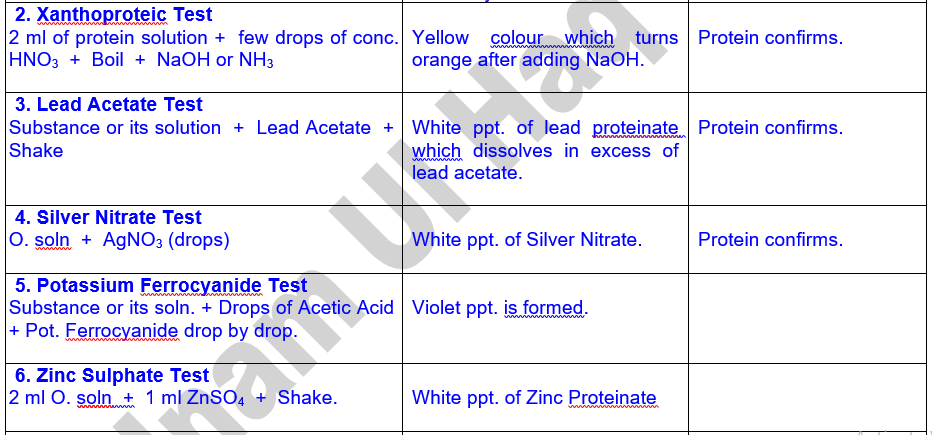
Tests
for Proteins
Tests for Fats and Oils
Model
Paper # 1 on Biochemical Tests (Analysis of glucose)
Object Identify
carbohydrates /proteins/ fats or oils in given sample of organic compounds.