Atomic Number (Z) or
Proton Number
Definition
Atoms of each element contain a characteristic number of
protons. In fact, the
number of protons determines what atom we are looking at (e.g., all atoms with
six protons are carbon atoms); the number of protons in an atom is called
the atomic number. In contrast, the number of neutrons for a given element
can vary to produce isotopes. The number of electrons can also be
different in atoms of the same element, thus producing ions (charged atoms).
For instance, iron, Fe, can exist in its neutral state, or in the +2 and +3
ionic states.
“The number of protons present in the nucleus of an atom is
called Atomic Number (denoted as Z)”. For
this reason, it's sometimes called the proton number.
OR
“The number of electrons revolving in the orbits of neutral atom
is called Atomic Number (as neutral atoms of an element contain an equal number of
protons and electrons).
Atomic Number (Z) = Number of Protons (P) = Number of electrons (e)
All the atoms of a particular element have the same number of protons, and hence the same atomic number but atoms of different elements have different atomic numbers.
For example, all carbon
atoms have the atomic number of 6, whereas all atoms of Oxygen
have 8 protons in their nucleus.
Representation
Atomic number is written as SUBSCRIPT on the LEFT HAND SIDE of the chemical symbol
of element. e.g. 3Li, 6C, 7N
etc.
Origin of Symbol Z
“Atomic number" in German is "Atomzahl", so the Z symbol for atomic number probably comes from "Zahl" (meaning number).
The letter Z is one of the signs for the highest god in Greek
mythology, Zeus.
In modern physics Z represents the greatest energy, nuclear power,
in its potential form, nuclear charge."
Example
1. Atomic number of hydrogen is 1 because its nucleus contains 1 proton.
2.Atomic
number of chlorine is 17 owing to the presence of 17 protons.
Range of Atomic Number
Because protons are units of matter, atomic numbers are always whole numbers. At present, they range from 1 (for hydrogen) to 118 (oganesson; the number of the heaviest known element).
Atomic number and mass number are always whole numbers because
they are obtained by counting whole objects (protons, neutrons, and
electrons).
Importance
1. It identifies the element i.e. it distinguishes one element from
another. The proton number is unique to each element so no two
elements have the same number of protons. Electrons come and go during chemical
processes but the proton number doesn’t change.
2. The modern periodic table is organized
according to increasing atomic number. Thus it determines the position of the element on the Periodic Table.
3. It is a key factor in determining the properties of an element. (Note, however, the number of valence electrons determines chemical bonding behavior).
Atomic Mass Number (A) or Nucleon Number
Definition
The sum of protons and neutrons in the nucleus of an atom is
called Mass Number or Nucleon Number denoted as “A”.
OR
the total
number of "nucleons" (Protons and Neutrons) in the nucleus of an atom
is called mass number. (protons and
neutrons are collectively called nucleons).
Mass Number (A) = number of protons (P) + number
of neutrons(n)
OR
Mass Number (A) = Atomic Number (Z) + number
of neutrons (n)
And
No of
neutrons= Mass number (A) – atomic
number (Z)
e.g.
Mass number of Na is 23 because its nucleus
contains 11 protons and 12 neutrons.
The nucleon number minus the
proton number gives you the number of neutrons of an atom.
Representation
Mass
number is written as superscript on the left hand side of the
chemical symbol of element. e.g. 12C, 14N
Notation
of Atom
To write the notation
of an atom, we need to know the symbol of the element, the atomic number and
the mass number. The mass number of the atom goes
above the symbol (superscript) and the atomic number is written as a
subscript below they symbol.
Calculating PEN
(Protons-Electrons-Neutrons) Numbers
1. The atomic number
is equal to the number of protons in an atom.
2. Since atoms are neutral,
then it is also the same as the number of electrons.
3. The mass number
is the number of protons plus neutrons.
4. The number of neutrons can thus be
calculated by subtracting the atomic number from the mass number.
Example
Beryllium for example has an atomic mass of 4, therefore it has 4
protons and 4 electrons.
The mass number of beryllium is 9, so it has 9 – 4 = 5 neutrons.
The PEN numbers for beryllium are thus:
p = 4
e = 4
n = 9 – 4 = 5
Isotopes of Elements
Definition
The
existence of isotopes of elements was first discovered by J.J. Thomson in 1913. The name of isotope was
introduced by Soddy because they have the same atomic number and hence
occupied the same place in the periodic table. (Isotope is a Greek word; iso =
same; topos = place). Nearly all elements found in nature are mixture of
several isotopes.
“Isotopes are atoms of the
same element having same atomic number
but different mass numbers
(atomic masses)”.
OR
“isotopes are different forms of
atoms of an element which have same
number of protons (and also electrons) but different number of neutrons in their respective nuclei”.
For example carbon has three of isotopes namely carbon-12, carbon-13 and carbon-14.
Different
isotopes of an element have same chemical
properties due to their identical electronic configuration (i.e. same
number of electrons in the shells) but they have different
physical properties because of their different
atomic masses.
Since the proton count
establishes elemental identity, chemical traits of different isotopes of the
same element tend to be the same. However, isotopes differ in respects of physical
properties which depend on atomic mass.
The different number
of neutrons, however, affects the stability and mass of the nucleus,
sometimes creating a radioactive isotope, sometimes creating a non-radioactive
(or stable) isotope.
Uses
of Isotopes
1.As tracers in physical, chemical, biological, medical and metallurgical researches.
2.As therapeutic agent for diagnoses and treatments of various diseases like cancer as radioactive isotopes are easily detected and traced.
3.Electrical power generation.
Symbolic
Representation of isotopes
The symbol for an isotope is
the chemical symbol (or word showing name of element) followed by a dash and
then the mass number. So C-14 is the isotope of carbon which contains 6
protons, 6 electrons and 14 – 6 = 8 neutrons. It can also be written as 14C.
In denoting particular isotopes of an element, the
following notation has been internationally adopted. The symbol of the element
is written with atomic at the head and atomic number at the bottom.
Alternatively, the name of the element is followed by the atomic with a hyphen
(-) in between. Thus the isotopes of carbon with atomic number 6 having atomic
masses of 12 and 14 may be written as:
12C or carbon-12 or C-12 reads ‘carbon twelve’ meaning
isotope of carbon with a mass of approximately 12 amu.
Kinds
of Isotopes
1. Natural non-radioactive
2. Natural Radioactive
3. Artificial Radioactive (made by neutron
bombardment)
Classification of elements on the basis of number of isotopes
Important
Points
1. Nearly all elements found in nature are mixture of several isotopes.
2. There are 287 different isotopic species in nature.
3. Out of 92 elements, 23 elements have
no isotopes, each consisting of only one kind of atoms e.g. 4Be,9F, 11Na, 13Al, 15P, 21Sc, 25Mn,
27Co, 33As, 39Y, 41Nb (niobium), 45Rh
(rhodium), 53I, 55Cs, 59Pr (praseodymium), 65Tb (terbium),
69Tm (thulium), 67Ho (Holmium), 79Gold (Au), 83Bi,
87Fr, Actinum(89Ac),
90Th, Protactinium (91Pa). The remaining elements
have 2 to 10 isotopes each.
NOTE
It is strictly improper to refer to elements that
exist in only one atomic form as having “one isotope”; actually such elements
like Be, F, Na, Al, P, Sc, Mn, Co, As, Y, Nb, Rh, I, Cs, Pr, Tb, Ho, Tm, Bi,
Ac, Th, Pa have no isotopes i.e. they have no other atomic form that
is like them in all respects except mass. The term isotopes require the
existence of at least two atomic forms of an element; in the same sense that
the word twin requires the existence of a pair. Recently the term mono-isotopic
is evolved for elements found in nature as a single atomic (isotopic) form.
4. The heavier isotopes of elements usually occur very rarely in the atomic population (e.g. 1 part in 4500 for 2H, 1 part in 140 for U-235; in the exceptional case of chlorine, the ratio of isotopes 35 and 37 is about 3:1).
5. The isotopes with even atomic number and even atomic masses are more abundant in nature.
6.Different isotopes of an element occur in different amount or isotopic abundances.
7. To work out the relative atomic mass of
an element, we need to find the average mass of all its isotopes which involves two steps:
(i) Multiply
each relative isotopic mass by its isotopic abundance and add up the results.
(ii) Divide
the result by the sum of the abundances. (If the abundances are given as percentages,
this will be 100).
Contrary to Dalton’s atomic theory, all atoms of a given element are not necessarily identical. In fact, most elements have been shown to be composed of two or more types of atoms mixed in a fixed proportion.
(i) The different atoms of such an element contain equal number of protons and, therefore, have the same atomic number.
(ii) The atoms which vary from one have different
number of neutrons in the nucleus. Thus they have different atomic masses.
Isotopes of Hydrogen
There are three isotopes of hydrogen namely protium or ordinary hydrogen, deuterium or heavy hydrogen, tritium or radioactive hydrogen. Protium is by far the most abundant in natural hydrogen, deuterium about 0.0156 % and tritium only one out of 10, 000, 000 hydrogen atoms.
The atomic number of three isotopes of hydrogen is 1 while their mass numbers are 1, 2 and 3 respectively. Each of the three isotopes of hydrogen has one proton in the nucleus and one extra-nuclear electron. The nucleus of protium contains one proton only while the number of neutrons in deuterium and tritium is 1 and 2 respectively.
Isotopes of Carbon
There are two stable isotopes and one radioactive
isotope of carbon. The carbon-12 contain 6 proton and 6 neutron, Carbon-13
possess 6 proton and 7 neutron, carbon-14 contain 6 proton and 8 neutron.
Carbon 12 is the most abundant (98.889%) isotope.
Isotopes of Oxygen
Oxygen has three isotopes;
with the relative abundances of 99.759, 0.037
and 0.204 respectively.
The
atomic number of each of these three isotopes of oxygen is 8 while their mass
numbers are 16,17 and 18 respectively. Thus each of these isotopes has 8 extra
nuclear electrons and 8 protons in the nucleus. The number of neutrons in O-16,
O -17 and O -18 is 8, 9 and 10 respectively.
Isotopes of Neon
Neon has three isotopes;
with their percentage abundance
99.92, 0.257 and 8.82% respectively.
The
atomic number of each of these three isotopes of neon is 10 while their mass
numbers are 20, 21 and 22 respectively. Thus each of these isotopes has 10
extra nuclear electrons and ten protons in the nucleus. The number of neutrons
in Ne-20, Ne-21 and Ne-22 is 10, 11 and 12 respectively.
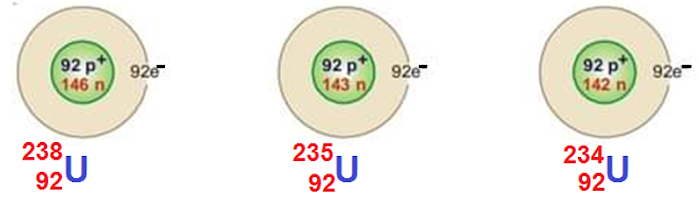
Isotopes of Uranium
There are three common isotopes of uranium with
atomic number 92 and mass number 234, 235 and 238 respectively. The uranium is
found 99% in nature.

Elements with no Isotopes or Mono-isotopic Elements
Nearly all elements found in nature are mixture of several isotopes. Out
of 92 natural elements, only 23 elements have no isotopes, each consisting of
only one kind of atom. The remaining 69 natural elements have from 2 to 10
isotopes.
Matter and its State
Definition
Anything
that exists is matter. Matter is the stuff of which the universe is made.
Scientifically anything which possesses mass and occupies space (volume) is
called matter. i.e. any species having mass and occupying space is known as
matter e.g. air, wood, water etc.
States
of Matter
The
different states of matter are due to difference of energy in increasing order.
There are four states of matter:
1.Gaseous State (Simplest form of matter)
2.Liquid State (Least abundant
form of matter)
3.Solid State (Most abundant form of matter)
4.Plasma (Most
abundant state of matter in the universe)
Only plasma
is not the phase transition state of matter
Classification of Matter

Substance and Impure Substance
Matter is classified as pure substance and impure substances (mixtures) at the bulk level or macroscopic level:
Substance or Pure Substance
1. A piece of matter in pure form is called a substance. A sample of pure matter whose composition is uniform throughout is called a substance. Pure substances have fixed composition and their constituents cannot be separated by using simple physical methods of separation.
2. It has a fixed composition and specific properties.
3. It is a type of substance which cannot be separated into more than one type of components by physical methods having same properties throughout their bulk.
4. Every substance has physical and chemical properties.
5. They are made up of one kind of matter.
6. Elements and compounds are the examples of pure substances. An element is composed of one type of particles which could be either be atoms or molecules. Na, Mg, Cu, Ag, S, F, P etc. have only one types of atoms. A compound is formed by the combination of two or more atoms of different elements. For example H2O, CO2, NH3, CH4 etc.
Examples
Tin,
sulphur, diamond, water, pure sugar (sucrose), table salt (sodium chloride),
baking soda (sodium bicarbonate) etc.
Impure Substances
It is a type
of substance which can be separated into their components by physical methods.
Examples
The
only example of impure substance is mixtures.
Element
The identical atoms with same atomic number unite to form an element and different elements combine together to form compounds. Therefore, elements are the simplest substances that we can use and investigate in chemistry because an element cannot be split into other substances (unlike compounds).
An element is a pure substance made up of only one type of atoms (unlike compounds) which cannot be further divided (split) into simpler substances by ordinary chemical means in which all the atoms are chemically identical having same atomic number. For example; Gold is an element and if it is broken into small pieces, each piece will retain the properties of gold.
An element is a pure substance made up of same type of atoms with same atomic number and cannot be decomposed into simpler substances by ordinary chemical reactions.
Elements may exist as atoms like the Noble Gases e.g. helium He or as molecules e.g. hydrogen H2 or sulphur S8.
Examples of
some elements
Gaseous Elements; Hydrogen, Oxygen, Nitrogen, Fluorine, Chlorine, Helium, Neon, Argon, Kr, Xe, Rn etc.
Liquid Elements; Bromine, Mercury.
Solid Elements; All metallic elements (e.g. Na, K, Al) & some non-metals (C, S, I, P)
Natural
Abundance/% Abundance of Common Elements in Earth’s Crust in % by Mass
[Oxygen
abundance is 50% which means that in a 100 g sample of Earth’s crust, there are
50 g of the element oxygen.]
Natural Abundance of Elements in Human Body
Total number of elements
There
are 118 elements, out of which 92 are naturally occurring elements called
Natural Elements while the rest (26) are man-made or Synthetic Elements.
Trans-Uranium
Elements or Trans-uranic Elements
The
elements with atomic number greater than 92 are called Trans-Uranium
Elements e.g. Am, Es
Classification of Natural Elements
The 92 naturally occurring elements can be divided into three groups:
Metals; (70 in numbers or 87 in numbers out of 112 elements).
Non-metal; (17 in numbers).
Metalloids; (8 in numbers).
|
|
Difference
b/w Element and Compounds
Metal
Definition
A metal (Greek; metallon) is an electropositive element that readily forms positive ions by losing their one or more valence electrons and in which its atoms are held together by metallic bonds. The metals are sometimes described as a lattice of positive ions (cations) in a cloud of free valence electrons (electron sea) i.e. in metals, positively charged metal ions are fixed in a crystal lattice in a sea of delocalized mobile valence electrons.
Metals are the solid (except mercury which is liquid) lustrous elements which are malleable and ductile having high density and high tensile strength and are good conductor of heat and electricity.
Elements
of group IA are called Alkali metals.
Elements
of group IIA are called Alkaline earth metals.
Metals
of A - Group family are shown in table 8.2
► Most abundant metal is Al.
► Most useable metal is Fe.
► Most reactive metal is Cs
► The lightest metal is Li
► The heaviest metal is Os
► Most malleable, ductile metals are gold, and silver.
Types of Metals
Metals have been subdivided into:
1. Normal or representative metals
2. Transition metals
1. Normal or Representative Metals
They
belong to A groups of the periodic
table having 1 to 5 valence electrons
forming white compounds. They are total 19 in numbers.
2. Transition metals
They
belong to B groups of the periodic table having 3 to 8 valence
electrons forming coloured compounds. They are total 68
in numbers. They are further divided into d-Block metals (40 in numbers) and
f-Block elements (28 in numbers).
Outer
Transition metals or d-Block
Elements (40 in numbers)
Physical and Chemical Properties of Metals
Physical properties
1. Physical State2. Hardness
3. High density
4. Metallic luster
5. opaque nature
6. High tensile strength
7. Malleable
8. ductile
9. High m.p & b.p.
10. High conductivity
11. Magnetic behaviour
12. Alloy formation
13. Position in periodic table
14. Total number
1. Solid
Physical State
All metals are solid at room temperature except Hg,
Cs and Ga.
2. Hardness
They are hard except Na and K which are soft and can
be cut with a knife.
3.
High density
They have high density and usually more denser than
water except Li, Na, K.
4. Metallic lustre
They have characteristic shiny metallic lustre
(shine) on their surface. (especially when cut).
5. opaque
nature
They are opaque (light cannot pass through them).
6. High tensile strength
They have high tensile strength i.e. they are tough
and strong.
7. Malleable
They are malleable (stretchable or dentable) i.e.
hammered into sheets
8. ductile
They are ductile (flexible) i.e. hammered into
sheets
9. High m.p & b.p.
They have high melting and boiling points.
10. High conductivity
They are good conductor of heat and electricity.
11. Magnetic
behaviour
Most of the metals are paramagnetic i.e.
attracted in a magnetic field.
12. Alloy formation
Metals form alloys when mixes with each other.
13. Position in periodic table
Metals are found on the left side of the periodic
table. In the periodic table, elements of group IA, IIA and all transition
elements are metals. Some of the elements of group IIIA (Al, Ga, In, Tl), IVA
(Sn, Pb) VA (Bi) are also metals.
14. Total number
The majority of elements are metals about 80% (about 95 in numbers) or three
fourth (3/4) of the known elements are metals.
(However, non-metals elements are more abundant in nature). Out of 92 natural
elements, 68 are metals. Only about 19 are definitely
non-metals but about 7 more are semi-metals (metalloids)
of mixed physical and chemical character.
Chemical properties
1. Forming Positive ions2. Metallic Bonding
3. Fewer no. of valence electrons
4. Positive Oxidation state
5. Low ionization energy
6. Reducing agent
7. Basic Nature of oxide
8. Basic Nature of hydrides
9. Action of water
10. Action of dilute acids
11. Action of alkalis
12. Action of air
1. Forming
Positive ions
Metals are electropositive elements and thus they act as electron
donor and readily form positive ions by losing their valence electrons typically
attaining noble gas electronic configuration due to their low ionization energy.
2. Metallic
Bonding
Metals atoms or ions are held together by metallic bonding.
3. No. of
valence electron
Most of the metals have less than 4 valence electrons except some transition metals which may have more than 4 valence
electrons (many metals have only one or two valence electrons).
4. Oxidation
state
They always exhibit positive oxidation state ranging from +1 to +7 (may be zero or even fractional & +8 for Os
and Ru).
5. Low ionization energy
They have low I.P. values
due to their large atomic
size and less nuclear charge
which lead to their strong electropositive character.
6. Reducing agent
They are always reducing agent.
7. Basic
Nature of oxide
They mostly form basic oxides e.g. Na2O,
Li2O, CaO, MgO, BaO, Na2O2, etc. except some
transition metal oxides which may form either form acidic (CrO3, Mn2O7
etc.) or amphoteric (ZnO, Cr2O3) or some normal metals
oxides (BeO, Al2O3, PbO, PbO2, SnO, SnO2).
Na2O + H2O ------> 2NaOH
8. Basic
Nature of hydrides
They form mostly stable basic hydrides except transition metals which form interstitial
hydrides.
9. Action of
water
Many metals dissolve chemically in water at different temperature
evolving H2 gas. Iron, Zinc, magnesium react only with steam to
produce respective oxide and H2 gas while all other metals react
with cold water producing corresponding alkali liberating H2 gas.
10. Action
of dilute acids
Dilute acids dissolve most of the metals (except Cu,
Ag, Au, Pt, Pb etc.) to produce salt
and H2 gas.
M(s) + 2H+ -----> M2+(aq) + H2
11. Action
of alkalis
Most of the metals are unaffected by alkalis.
Amphoteric metals like Al, Zn, Sn etc. dissolves in alkalis forming their
respective oxysalt evolving H2 gas.
Zn(s) +
2NaOH -----> Na2ZnO2aq) + H2
12. Action of air
Most of the metals corrode in air giving
their respective oxides.
Non-metal
Definition
A
non-metal is an electronegative element that readily forms negative ions by
gaining one or more electrons and in which its atoms or molecules are held
together either by covalent bonds or van der Waal’s forces.
Non-metals
are non-lustrous (dull) elements which are brittle i.e. non-malleable and
non-ductile having low density and low tensile strength and are bad conductor
of heat and electricity (except graphite) found in all the three states of
matter (mostly gases or solids except bromine which is a volatile liquid).
Examples of Non-Metals
Gases; H, O, N, F, Cl, He, Ne, Ar, Kr, Xe, Rn
Liquid; Br
Solids; C, P, S, Se, I
Physical and Chemical Properties of Non-metals
Physical Properties
1. Occurrence in all three Physical State
2. Hardness
3. Low density
4. Lack of Metallic luster
5. opaque nature
6. Low tensile strength
7. Non-malleable
8. Non-ductile
9. Low m.p & b.p.
10. Low conductivity
11. Non-magnetic behaviour
12. Position in periodic table
13. Alloy formation
14. Total number
1. Physical
State
They are found in all the three states of matter.
2. Hardness
Solid non-metals are soft and brittle except diamond (hardest natural element known).
3. Low
density
They have low density and are lighter than metals. However all of them are more denser than water.
4. Lack of
Metallic luster
They lack metallic luster and usually
they are dull except diamond, graphite, Si and iodine.
5. opaque nature
Solid non-metals are opaque. However gaseous non-metals are transparent and
light can pass through them.
6. Low
tensile strength
They low high tensile strength
7.
Non-malleable
They are brittle and thus non-malleable i.e. cannot
be hammered into sheets.
8.
Non-ductile
They are non-ductile i.e. cannot be hammered into wires.
9. Low m.p & b.p.
They have low melting and boiling
points except carbon (3350°C).
10. Low
conductivity
They are poor conductor of heat and
electricity except graphite (super conductor).
11.
Non-magnetic behaviour
Most of the non-metals are non-magnetic.
12. Position
in periodic table
Non-metals are found on the right
side of the periodic table. In the periodic
table, majority of the elements of p-Block i.e. group IVA(C), VA (N, P) VIA (O,
S, Se), VIIA (F, Cl, Br, I) and VIIIA (He, Ne, Ar, Kr, Xe, Rn) are also
non-metals.
13. Alloy
formation
They do not form alloys with each other. However some non-metals like C,
P, Si form alloys with metals.
14. Total
number
Very few elements are non-metals. i.e. about 20% (17 in numbers) or 1/5th
of the known elements are non-metals.
However non-metallic elements are more abundant in nature. e.g O constitutes
about 50% of the earth crust.
Chemical Properties
1. Forming Positive ions
2. Bonding
3. Greater no. of valence electron
4. Showing negative and positive Oxidation state
5. High Electron affinity
6. Oxidizing agent
7. Variable Nature of oxide
8. Variable Nature of hydrides
9. Action of water
10. Action of dilute acids
11. Action of air
12. Action of alkalis
1. Forming Positive ions
2. Bonding
Non-metals atoms or molecules are held
together either by covalent bonds or van der Waal’s forces.
3. Greater
No. of valence electron
Most of the non-metals have more than 3 valence electrons (except He
which has only 2) ranging from 4 to 7 (except all noble gases having 8 except
He).
4. Oxidation
state
They exhibit variety of oxidation states (which may be negative,
positive, zero or even fractional) ranging from -1/2 to +7.
5. High
Electron affinity
They have high electron affinity values due to their small atomic size
and greater nuclear charge which lead to their strong electronegative
character.
6. Oxidizing
agent
They are always oxidizing agent (except
hydrogen and carbon).
7. Acidic
Nature of oxide
They mostly form acidic oxides. However some
non-metallic oxides may be neutral (CO, NO, N2O, H2O).
CO2
+ H2O -----> H2CO3
SO2
+ H2O -----> H2SO3
8. Variable Nature of hydrides
Their hydrides are either neutral (CH4),
basic (NH3) or acidic (HF, HCl, HBr)
9. Action of
water
Non-metals in general do not react with cold or hot
water. However, red hot carbon reacts with steam at elevated temperature to
form water gas.
C + H2O -----> CO + H2
10. Action
of dilute acids
Non-metals are generally inert toward
dilute acids.
11. Action
of air
Non-metals are generally not affected by cold-dry air. However, when they
ignited in air, they react with oxygen of air forming respective oxides which
are acidic in nature.
12. Action
of alkalis
Non-metals are generally inert toward
alkalis
Metalloids or Semi-metals
Definition
Metalloids
are the elements which exhibit dual character and have characteristics
of both metals as well as non-metals. i.e. have a blend of metal and
non-metal properties. e.g. silicon appears lustrous (a feature of metals) but
is not malleable or ductile rather it is brittle (a characteristic of
non-metals) and also it is a semi-conductor. Their oxides are amphoteric
showing acidic as well as basic nature.
Many
metalloids show intermediate properties between the metals and
non-metals. They have varying ability to conduct electricity depending on
temperature, exposure to light or presence of small amount of impurities. Some
of the metalloids are semi-conductors like silicon, germanium and boron.
Position
of metalloids in the periodic table
Metalloids are found along stair-step (staircase) line or diagonal boundary between metals and non-metals from B to Al to the border between Po and At (the only exception to this is Al which is classified under “weak or other metal’).
Examples of Metalloids
There are total 8 metalloids:
1. Boron of group IIIA
2. Silicon and germanium of group IVA
3. Arsenic and antimony of group VA
4. Tellurium and polonium of group VIA
5. Astatine of group VIIA
Symbol
The
short hand representation used for the full name of an element is called
Symbol. Thus a symbol is an abbreviation for the chemical name of an
elements representing only one atom of the elements.
1. A symbol is taken
from the English, Latin, Greek and German name of elements.
2. Symbols are usually
one or two letter long.
3. Every symbol starts
with capital letter as carbon with C or sulphur as S.
4. If symbol is second letter then start
with capital and second will be in small letter as He for helium, Na for sodium, Cr for chromium.
Examples
1. Usually, the first letter of the English name of the element (in capital) is taken as its symbol.
e.g.
2. Sometimes the first two initial letters of the name of an element is taken as symbol, the initial letter being capitalised
e.g.
3.
The symbols of some elements are derived from their Latin name.
e.g.
Compounds
Compounds are pure substances which consist of two or more
elements chemically
combined in a fixed proportion of their atoms or mass and cannot be
separated by physical methods.
They are always homogenous i.e. their constituents cannot be seen as separate
particles even by high power microscope.
Examples
Sodium Hydroxide (NaOH)
Methane (CH4)
Calcium Carbonate (CaCO3)
Explanation
1. In the
formation of a compound, there is always a chemical change between the
components element, so that the compound formed is a new substance.
2.In
a compound, the constituent elements lose their characteristic properties.
Thus a compound always possesses properties entirely different from those of
their constituent elements. e.g.
(i) Zinc
is a grey solid and sulphur is yellow solid while their compound zinc sulphide
is white.
(ii) Carbon dioxide (CO2) is a compound which neither burns nor helps in burning, while its constituent carbon itself burns and oxygen helps in burning.
3. The compound is formed by the fixed ratio of atoms of component elements, e.g. water is a compound of hydrogen and oxygen is which H and O are present in the ratio of 2:1 by atoms.
4.
The melting and boiling points of
compounds are sharp.
Examples
Types of Compound According to bonding
1. Covalent or Molecular compounds; comprising of molecules in which atoms are covalently bonded.
2. Ionic or Electrovalent compounds; comprising of aggregate of cations and anions in crystal lattice
Types of Compound According to Origin
1. Inorganic compounds; Compounds of all elements except carbon, also contain C in special forms.
2. Organic compounds; Covalent compounds of carbon , mostly hydrocarbons and their derivatives
Types of Compound According to Taste
1. Acids; Containing ionizable H+ ions. e.g. HCl, HBr, HI, HNO3, H2SO4, CH3COOH etc.
2. Bases; containing ionizable OH- ions. e.g. NaOH, KOH, Ca(OH)2, Ba(OH)2 etc.
3. Salts; Acid-base neutralization product. e.g. NaCl, KCl, NaBr, NaI, KBr, KI etc.
Types of Compound According to Number of elements
1. Binary compounds; comprising of only two different elements.
2. Ternary compounds; comprising of three or more elements.
Types of Compound According to Solubility
1. Soluble compounds
2. Sparingly soluble compounds
3. Insoluble compounds
Types of Compound According to Conductivity
1. Electrolytes
2. Non-electrolytes
Mixtures
A mixture is an impure substance which consists
of two or more pure substances (element/compound) which are united
physically in the variable ratio.
They do not have uniform composition.
The
components making up the mixture retain their original properties so that
nothing new thing is formed. A mixture can be separated into its components by
simple physical methods. The melting and boiling points of mixture are not
sharp.
Types of Mixtures
There are two main types of mixtures
1. Homogenous Mixtures
1.They have uniform composition
2. In a homogenous mixture all the substances are evenly distributed throughout the mixture. Their components cannot be seen with naked eyes.
3. They are also known as solutions or alloys.
e.g.
air, aqueous sugar solution
etc.
2. Heterogeneous Mixtures
1. Mixtures which do not have uniform
composition throughout their mass are called Heterogeneous Mixtures.
2. In a heterogeneous mixture the substances are not evenly distributed.
e.g.
soil, rocks, ice cream, chocolate chip cookies, pizza, rocks etc.
Examples
Valency
Old Definition
Valency of
an element is defined as the number which expresses the combining or displacing
tendency of an element with other elements”. “Valency may also be defined as
the number of hydrogen atoms which combine with or displace one atom of an
element”. Valency is a simple whole number.
valency of chlorine is one as it combines with one H atom to form HCl and valency of oxygen is two as it combines with two H atoms to form H2O.
Modern Definitions
Valency is defined as the number of electrons lost
or gained by an atom of the element during a chemical reaction in order to
complete its outermost shell (Octet)”.
For example
valency
of calcium is 2 because it loses two electrons to form Ca2+
ion. Similarly valency of oxygen is also
2 as it accepts two electrons to form O2‒ ion.
Hund’s Rule Definition
The number of unpaired electrons or partially filled orbitals constitutes the valency of an element.
For example;
Nitrogen has 3 unpaired electrons in its valence shell, so its valency is 3.
Variable Valency
The elements having variable valencies are shown below:
The element that exhibits lower valency will be suffixed with “ous”. While the element that exhibits higher valency will be suffixed with “ic”.
Significance
The formula of compounds can be found out by means of valencies of two combining atoms.
Explanation
1. Valency is simply a whole number
without positive or negative sign.
2. The valency of elements ranges 1 to
7. Valency of an element cannot exceed
7.
3. Valency of an element cannot be zero
except noble gases.
4. Valency of an element cannot be in fraction.
Types of elements on the basis of valency
Atom
Definition
All matter is composed of tiny entities called atom. An atom is the smallest neutral particle and basic units of an element that can enter into chemical combination and define structure of elements. Thus atoms are building blocks of element (matter). An element consists of only one kind of atoms.
Historical Background
Evidence of Atom
Atom is a very small particle and it is
not possible to see an atom but evidence of its presence in an element can be
seen by the following ways:
(i) Electron
Microscopy
In electron microscope, the beam of
electrons is used. The figure show electron microscopic photograph of a piece
of a graphite magnified about 15 million time. The bright band in the figure
are layers of carbon atoms.
(ii) X-rays
Diffraction method
X-rays diffraction pattern obtained from diffractrometer has made us able to believe the existence of an atom.
Characteristics of Atom
1.
An atom is electrically neutral
because it contains equal number of positively charged particles (protons)
and negatively charged particles (electrons). i.e. atoms are neutral and they
have no charge overall (unlike ions which are atoms or group of atoms that have
lost or gained electrons and hence acquire charge). This is because they have
the same number of protons and electrons. (The charge on the electrons is the
same size as the charge of on the protons, but opposite – so the charges cancel
out giving electrically neutral atom). In an ion, the number of protons is not
equal to the number of electrons giving an overall charge. For example, an ion
with a 2- charge has two more electrons than protons.
[An atom was considered to be indivisible by Dalton but modern researches revealed that an atom is divisible and consists of more than 100 sub-atomic particles (electron, proton, neutron, hypron, neutrino, meson, muon, positron etc.) out of which 3 are called fundamental particles namely protons, neutrons (together called as nucleons) and electrons]
2. Atoms are very reactive and undergo chemical change readily.
3. Atoms may or may not exist independently or in free state.
4. With the exception of atoms of noble gases (e.g. Ne, Ar, Kr, Xe, Rn), all atoms have incomplete Octet (an octet means 8 electrons in valence shell), that is why atoms are unstable and can take part in a chemical reaction to acquire stability.
5. Na, Ca, C, S, O etc. are the
symbols of few atoms.
Composition of atom
1. The
atom is made up of three sub-atomic particles namely protons, neutrons and
electrons.
2. Protons
are heavy and positively charged.
3. Neutrons
are heavy and neutral.
4. Electrons
are lighter having hardly any mass and are negatively charged.
*NOT a nucleon. Electrons are arranged in energy levels or shells in orbit around the nucleus
Nucleus
1. It
is in the middle of the atom.
2. It
contains protons and neutrons.
3. It
has a positive charge because of the protons.
4. Almost
the whole mass of the atom is concentrated in the nucleus.
5. The
nucleus is tiny as compared to the overall size of the atom.
Electrons
1. Electrons
move around the nucleus in electron shells or orbits.
2. They
are negatively charged.
3. They
are tiny but their shells cover a lot of space.
4. The size the electrons shells determines the
size of the atom. Atoms have a radius (known as atomic radius) of about 10–10
m.
5.Electrons
have tiny negligible mas (so small that it is sometimes given as zero).
Protons and neutrons are much heavier than electrons. You can think of the mass of an electron as about 1/2000th of the mass of a proton or neutron, so, a pretty small mass BUT they occupy most of the space of an atom!!! You should also realize because of the relatively small mass of the electrons most of an atom's mass is in the nucleus. You see values of 1/1836 quoted for the relative mass of an electron, but don't worry about it, there are different ways/scales on which an electron's mass has been calculated.
The actual mass of a proton or neutrons is ~1.67 x 10–27 kg (~1.67 x 10–24 g)
The mass of an electron is ~9.1 x 10–31 kg (~9.1 x 10–28 g)
The mass of an atom varies from about 1 x 10–20 to 1 x 10–18 kg (1 x 10-23 to 1 x 10–21 g) depending on the element
The radius of the nucleus ranges from about 1 x 10–16 to 1 x 10–14 m (1 x 10–7 to 1 x 10–5 nm) depending on the element
The diameter of atoms varies from about 1 x 10–10 to 5 x 10–10 m (0.1 to 0.5 nm) depending on the element
Generally speaking the radius of an atom is about 10,000 times that of the nucleus!
A typical relatively small molecule would be no bigger than ~1 x 10–10 to 1 x 10–9 m (~0.1 to 1 nm)
Size of atom
According to X-rays study, the diameter
of an atom is of the order of 2 x 10–10 m (0.2 nm). If atoms are
joined together in a line, two million atoms will be required to cover a full
stop!
Molecule
Definition
Elements combine to form compounds which are of two types namely covalent and ionic compounds. All covalent or molecular compounds are composed of tiny and discrete entities called molecules.
Molecule is chemical combination of atoms.“A molecule is the smallest particle of a substance (element or covalent compound) which is aggregation of two or more than two similar or dissimilar atoms held together by covalent bonds that has the chemical properties of that element or Compound.”.
OR
“a molecule is a neutral, covalently bonded group or cluster of atoms that acts as a unit of covalent compounds retaining all the properties of the substance”. Thus molecules are building blocks of some molecular elements (e.g. H2, O2, N2, F2, Cl2, Br2, I2, P4, S8 etc.) and all covalent compounds.
A molecule is two or more atoms bonded together to form a single chemical entity. When atoms combine by forming covalent bonds, the resulting collection of atoms is called a molecule. We can therefore say that a molecule is the simplest unit of a covalent compound.
Characteristics of Molecule
1. A molecule is electrically neutral because it comprises of neutral atoms.
2. Molecules are relatively inert or unreactive.
3. Molecules can exist independently or in free state.
4. In molecules, each atom usually possesses complete octet. That is why molecules are stable and cannot undergo chemical change under normal conditions.
Types of molecules
Molecules are of two types:
Difference between atom and molecule
It consists of atoms of one chemical element. The molecules of an element contain the same type of atoms. The molecules of most of the non-metals are made up of more than one atom. The elements which consist of molecules are called molecular elements e.g. O2. Molecules of many elements are made up of only one atom of that element. e.g. noble gases like Argon (Ar), Helium (He) etc.
E.g.
a molecule of oxygen (O2)
consists of two atoms of oxygen and is known as a diatomic molecule.
Ozone (O3) consists of three
atoms of oxygen is known as triatomic molecules.
A molecule of phosphorus (P4) comprises of four atoms of phosphorus and is called tetraatomic molecule.
2. Heteroatomic/heteronuclear
Molecules/ Molecules of Compounds
a chemical compound composed of more than one element consists of two or more different atoms and are called heteroatomic molecules. e.g. H2O.
Atoms of different elements join together in definite proportions to form molecules of compounds.
Diatomic Molecules
A diatomic atom is composed of only two atoms, of the same or different chemical elements. Examples of diatomic molecules are O2, CO etc.
There are seven diatomic Elements. These seven elements are so reactive that they can be found very often bonded with another atom of the same type.
Hydrogen (H2)
Nitrogen (N2)
Oxygen (O2)
Fluorine (F2)
Chlorine (Cl2)
Iodine (I2)
Bromine (Br2)
Representing molecules;
chemical formulas
Chemical formulas, sometimes also
called molecular formulas, are the simplest way of representing molecules.
Molecules can be represented with a chemical formula which shows the
types of atoms in the molecule, and, uses subscripts to show how many of each
type of atom is present.
In a chemical formula, we use the
elemental symbols from the periodic table to indicate which elements are
present, and we use subscripts to indicate how many atoms of each element exist
within the molecule. For example, a single molecule of NH3, ammonia,
contains one nitrogen atom and three hydrogen atoms. By contrast, a single
molecule of N2H4, hydrazine, contains two nitrogen atoms
and four hydrogen atoms.
Representing molecules; structural formulas
Chemical formulas only tell us how many
atoms of each element are present in a molecule, but structural formulas also
give information about how the atoms are connected in space.
The structural formula of a chemical compound is a graphic
representation of the molecular structure, showing how the atoms are
arranged. In structural formulas, we actually draw the covalent bonds
connecting atoms.
Ion or Simple Radical or
Radical
Definition
The electrically charged atoms or group of atoms formed by
the loss or gain of electrons are called Ions. An atom or group of atoms that
carries an electric charge which is formed by the loss or gain of one or
more electrons is called an ion.
e.g.
Na+, NH4+, Cl−, CO32−.
The electrically charged
atoms are called Ions. An atom or group of atoms that carries an electric
charge either positive or negative behaving as an entity which is formed by the
loss or gain of one or more electrons is called an ion. This loss or gain of
electrons takes place to obtain a full outer shell of
electrons.
Ions are not electrically
neutral as in ion, number of protons and electrons are not equal. Thus an ion
is electrically charged because it contains different number of positively
charged particles (protons) and negatively charged particles (electrons).
Existence of ions
Ions exist in solution or
crystal lattice. Ions always exist ionic compounds only.
Effect of Light on Ions
Ions are not affected by
light
Complete Octet
Ions usually possess complete octet.
For instance Na+ or Cl− both contains 8 electrons in their valence shell.
The ions formed by normal
elements have complete octet while ions formed by transition metals have
incomplete octet (except Sc3+, Y3+, Ti4+, Cr6+,
V5+, W6+ etc.).
Characteristics
1.Ions are not electrically neutral as in ion, number of protons and electrons are not equal. Thus an ion is electrically charged because it contains different number of positively charged particles (protons) and negatively charged particles (electrons).
2. Ions always exist in ionic compounds only.
3. Ions usually
possess complete octet.
For instance Na+ or Cl- both contains 8 electrons in
their valence shell. The ions formed by
normal elements have complete octet while ions formed by transition metals have incomplete octet
(except Sc3+, Y3+, Ti4+, Cr6+, V5+,
W6+ etc.).
4. The electronic structure of ions of elements in Groups I, II,III, VI and VII will be the same as that of a noble gas (e.g. helium, neon, argon, krypton, xenon and radon).
5.All metals lose electrons to other atoms to become positively charged ions called cations.
6. All
non-metals gain electrons from other atoms to become negatively charged ions called anions.
7. When writing about ions, we use the notation 1-, 2+ etc. to describe the charge of the ion, with the number first followed by the sign (+/-). It is incorrect to write them the other way around like +1,-2 etc. as this refers to the oxidation state, not the charge.
8. group I (Li, Na, K): form 1+ ions, group II (Mg, Ca, Ba): form 2+ ions, group III: form 3+ ions
9. group V (N, P, As): form 3- ions, group VI (O, S, Se): form 2- ions, group VII: form 1- ions
10. ions from common oxyacids: NO3− (nitric acid), SO42− (sulfuric acid)
Naming Monoatomic Ions
1. To name monoatomic
or single element positive ions of representative elements of group A
like Na+, K+, Mg2+, etc. , write the name as
from the periodic table adding the word ion afterwards.
2. To name monoatomic
or single element negative ions like F–, O2–, P3–
etc., write the name from the periodic table replacing the ending with ide
adding the word ion after the name.
3. To name monoatomic
or single element positive ions of transition elements like Fe3+,
Cu2+, Co3+ etc., write the ionic charge (1+, 2+, 3+ etc.)
as a Roman Numeral in parenthesis. So Cu2+ would be
the copper(II) ion. Fe3+ would be called iron(III) ion.
4. Some monoatomic
positive ions of non-transition elements or representative elements like
Pb, Sn, Sb, As are also named by writing their ionic charge as Roman Numeral in
parenthesis.
Types of Ions based on complexity
1.Simple Ion/simple radical/Monoatomic Ion;Na+, Cl–.
2.Compound ion (Polyatomic ions); NH4+, HCO3–.
3.Complex ion or complex radical;[Fe(CN) 6] 4¯, [Cu(NH3)4]2+
4. Molecular Ions; [NO+,
CO+]
Types of Ions according to Charge
There are two types of ions, cations and anions.
(a) Cations or metallic
ions or acid radicals, formed by loss of election e.g. Na+
(b) Anions or nonmetallic or basic radicals, formed by gain of
electron e.g. Cl–
Cation/Basic Radicals/Metallic Radicals
Definition
The positively charged ion
formed by the loss of electron by neutral metal atom containing more
protons than electrons is called Cation. Loss or removal of electron from
neutral metal atom gives cation. e.g.
Origin or derivation
They are mostly metallic
in nature except cationic non-metallic radicals like NH4+,
PH4+, NO2+, etc.
Reason of Calling basic
Radicals
They are called basic
radicals as they are originated from bases.
Reason of Calling Cations
They are called cations as
they move towards cathode (negative electrode) during electrolysis.
Relative size
The size of cation is smaller
than its parent atom.
Anion/Acid Radicals/Non-metallic Radicals
Definition
The negatively charged ion
formed by the gain of electron by neutral non-metallic atom containing
more electrons than protons is called Anion. Gain of electron by neutral atom
gives anion. e.g.
Origin or derivation
They are always non-metallic
in nature. Most of the anions contain oxygen and they are called oxyanions.
Reason of Calling Anions
They are called anions as
they move towards anode (positive electrode) during electrolysis.
Reason of Calling Acidic
Radicals
They are called acidic
radicals as they are originated from acids.
Relative size
The size of anion is larger
than its parent atom.
Formula Unit
Ionic compounds do not exist as
individual discrete molecules. In some crystalline compounds (NaCl,
KCl, CaF2 etc.) and in some covalent network solids, there are no
discrete molecules but the atoms are bounded to one another in a network structure as aggregate of positive and negative
ions. Such compounds are represented by their simplest or empirical formula which simply shows the relative number of atoms
of each component.
Formula unit is the
lowest whole number ratio of ions in an ionic compound. It expresses the
smallest collection of oppositely charged ions that would be neutral in an
ionic compound or an ionic crystal lattice i.e. it is the lowest ratio of ions
represented in an ionic compound.
[Note;
the formula unit is analogous to molecule in a molecular compounds].
A formula unit in chemistry is the empirical formula of an ionic or covalent network
solid
compound used as an independent entity for stoichiometric calculations. A formula unit shows the
kinds and numbers of atoms in the smallest representative unit of a substance.
Examples include ionic compounds like
NaCl and K2O and covalent networks compounds such as SiO2
and C (as diamond or graphite).
A formula unit is
electrically neutral as it contains oppositely charged ions (cations and
anions) in lowest possible whole-number ratio so that the sum of charges of
ions becomes zero.
chemical
species
If one molecule is identical to another we can say
they are the same chemical species. Chemical species is a chemical entity, such
as particular atom, molecule, formula unit, ion (anions, cations), Molecular
ions and Free Radicals.
Molecular ions
A molecular ion is an
ionized molecule formed by the removal or addition of only electrons.
These ions can be generated by passing high energy electron beam or
alpha-particles through a gas. when
a molecule loses or gains electrons is called molecular ions.
For example
CH4+ N2+,
CO+, CO2+, O2+ etc.
Molecular ions also possess positive or negative charge like any ion.
If Molecular ion has negative charge it is known as anionic molecular ion, if it has positive charge then it is known as cationic molecular ion.
Cationic
molecular ions are more abundant.
Free Radicals
Definition
Free radicals are atoms and group of atoms having an
unpaired electron or uneven number of electron.
Representation
It is represented by putting a dot over the symbol of an element.
For example: Ho, Clo,
Existence
Free radical exist in
solution as well as in air
Effect
of Light
Free radical forms in
the presence of light.
Formation
Free radicals are formed when homolytic (equal)
breakage of the bond between two atoms takes place by the absorption of heat or
light energy.
Cl
– Cl → Clo + Clo
CH4
→ H3Co + Ho
Reactivity
Free radical is very reactive chemical species.
Even though have unpaired electrons, by convention, metals and their ions or complexes with unpaired electrons are not free radicals.
Types of Free Radicals
Most organic radicals
are quite unstable and very reactive. There are two kinds of radicals,
1. neutral
free radicals and
2. charged
free radicals
Difference between atom
and molecule
Difference between atom
and ion