Conceptual
Chemistry
Practical
Manual XI
I. Simple Titrations
Contents
1. You are given N/10 solution of HCl. Find out normality &amount of NaOH in g/750 cm3.
2. You are given 0.25 N soln of KOH. Find out normality & amount of hydrous oxalic acid in g/500 cm3
3. You are given N/20 soln of H2SO4. Find out normality & amount of anhydrous Na2CO3 in g/250 cm3.
4. You are given N/15 solution of HNO3. Find Normality & amount of KOH solution in g/0.75 dm3
5. You are given 0.5 N soln. of KOH. Find Normality & amount of sulphuric acid solution in g/600 cm3
6. You are given 0.05 N solution of H2SO4. Find out Normality & amount of K2CO3 in g/900 ml
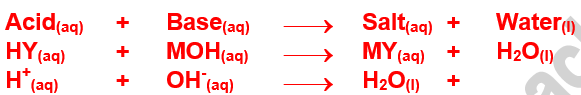
Acid base titration
using phenolphthalein
Object....Given _____ solution of _____, find out the Normality and Amount of ______ in g/___

Chemicals
Base ---------- ................ (Strong)
Indicator ----- Phenolphthalein
*write equation according to object.
Observations
Solution in burette (Titrant) = ______
Solution in conical Flask (Titrate) = ______
Normality of acid (N1) = ______ N
Volume of acid (V1) = 10.0 cm3
Normality of Base (N2) = ______N
Volume of Base (V2) = ____cm3 (Comes from burette reading)
Amount of NaOH in g/........... = _____ ?
Colour change = From Colourless to pink
Indicator used = Phenolphthalein
Burette Readings
|
|
Burette Reading Chart
Result
Normality of _______ solution = ............. N
Amount of _______ in g/......... = ............ g/_____
Acid base
titration using Methyl Orange
Object .... Given _____ solution of _____, find out the Normality and Amount of ______ in g/___
Chemicals
Observations
Burette Reading Chart
Result
Normality of _______ solution = ............. N
Amount of _______ in g/......... = ............ g/_____
Simple Titration (Experiment No. 1)
Object .... You are given N/10 (0.1 N) solution of HCl. Find out the normality and amount of NaOH in g/750 cm3.
Chemicals
Observations
Burette Reading Chart

Calculation
1. Normality Determination
2. Amount Determination
Result
Simple Titration (Experiment No. 2)
Object .... You are given 0.25 N solution of KOH. Find out the normality and amount of hydrous oxalic acid (H2C2O4.2H2O) in g/500 cm3.
Chemicals
Observations
Burette Reading Chart
Calculation
II. Redox Titrations
Contents
1. You are given N/10 solution of KMnO4. Find out normality & amount of FeSO4.7H2O
in g/750 ml.
2. You are given 0.25 N solution of KMnO4. Find out normality & amount of Mohr’s
salt in g/500 cm3
3. You are given 0.05 N soln of hydrous
oxalic acid .Find normality & amount of KMnO4 in g/950 cm3
4. You are given N/30 soln of FeSO4.7H2O.Find
out normality & amount of KMnO4 in g/850 ml
5. You are given N/25 soln. of KMnO4.Find
out normality & amount of hydrous oxalic acid in g/1.5 dm3
Redox titration General Description
Object
Given _____ solution of _____, find out the Normality and Amount of ______ in g/___
Chemicals
1.Oxidizing agent ....... Potassium
permanganate (KMnO4) solution.
2.Reducing agent ....... Hydrous
Ferrous Sulphate, or Mohr’s salt or oxalic acid
3. Indicator ................. KMnO4 itself behaves as an indicator.
4. Helper/Medium ...... Acidic
(dilute H2SO4).
Theory
1. Titration is the type of quantitative volumetric analysis in which burette and pipette are used to standardize a solution. It is the process by which the strength of unknown solution is determined by treating it with a definite volume of a standard solution in the presence of an indicator.
2. Oxidation is the process during which oxidation number of an element is increased due to loss of one or more electron while reduction is the process during which oxidation number of an element is decreased due to gain of one or more electrons.
3. Oxidizing agent is a substance which gains electrons during reaction and thus they oxidize other substance and itself get reduced. e.g. KMnO4, K2Cr2O7 etc. Reducing agent is a substance which loses electrons during reaction and thus they reduce other substance and itself get oxidized. e.g. FeSO4.7H2O, FeSO4.(NH4)2SO4.6H2O, H2C2O4.2H2O etc.
4.Both oxidation and reduction takes place simultaneously and such reactions are called Redox reactions.
5. The titration
between reducing agent and oxidizing agent in which one reactant is oxidized
and other is reduced is called Redox titration. In redox titration KMnO4
is always a Titrant (i.e. taken in the burette) while reducing agent is
titrate. The titration is carried out in acidic medium so dilute H2SO4
is also taken along with reducing agent in flask in equal amount. No external
indicator is used as KMnO4 itself acts as an internal indicator.
Equations
Method
1. Wash the burette, pipette and titration flask with water and rinse the pipette with reducing agent and burette with KMnO4.
2. Clamp the burette vertically in an iron stand and rinse the burette with Titrant (KMnO4) solution. Now fill it with Titrant (KMnO4) solution carefully using funnel upto the zero mark.
3. After rinsing pipette with titrate (reducing agent) solution, pipette out 10 ml (cm3) Titrate (reducing agent) solution into the conical flask and add equal volume or half test tube of H2SO4 into it.
4. Run Titrant (KMnO4)
solution from burette into flask drop-wise with constant shaking till a
permanent pinkish tinge just appears by a single drop of Titrant (KMnO4).
This is the end point. Note down the reading of the upper meniscus.
5. Now throw the content of
the flask and wash it with distilled water and repeat the above process of
titration till two similar readings (concordant readings) are obtained
6. Calculate normality,
equivalent weight & amount of required specie.
Observations
1. Solution in the burette (Titrant) = KMnO4
2.Solution in conical flask(Titrate) = Reducing agent + H2SO4 (10 ml each)
3. Colour change = From Colourless to pink
4. Normality of Reducing
agent (N2) = ?
5. Volume of Reducing agent (V2) = 10 ml
6. Normality of KMnO4 (N1) = …… N
7. Volume of KMnO4 (V1) = ?
8. Amount of Reducing agent in g/750 ml = ?
9. Indicator used = KMnO4
as self-indicator
Burette
Reading Chart
Result
Redox Titration (Experiment No. 1)
Object
Chemicals
1. Oxidizing agent –––– Potassium
permanganate (KMnO4) solution.
2. Reducing agent –––– Hydrous
Ferrous Sulphate (FeSO4.7H2O) solution.
3. Indicator –––– KMnO4 itself behaves as an indicator.
4. Helper / Medium –––– Acidic
(dilute H2SO4).
Theory
1. Titration is the type of quantitative volumetric analysis in which burette and pipette are used to standardize a solution. It is the process by which the strength of unknown solution is determined by treating it with a definite volume of a standard solution in the presence of an indicator.
2. Oxidation is the process during which oxidation number of an element is increased due to loss of one or more electron while reduction is the process during which oxidation number of an element is decreased due to gain of one or more electrons.
3. Oxidizing agent is a substance which gains electrons during reaction and thus they oxidize other substance and itself get reduced. e.g. KMnO4, K2Cr2O7 etc. Reducing agent is a substance which loses electrons during reaction and thus they reduce other substance and itself get oxidized. e.g. FeSO4.7H2O, FeSO4.(NH4)2SO4.6H2O, H2C2O4.2H2O etc
4. Both oxidation and reduction takes place simultaneously and such reactions are called Redox reactions.
5. The titration
between reducing agent and oxidizing agent in
which one reactant is oxidized
and other is reduced is called Redox titration. In redox titration KMnO4
is always a Titrant (i.e. taken in the burette) while reducing agent is titrate.
The titration is carried out in acidic medium so dilute H2SO4
is also taken along with reducing agent in flask in equal amount. No external
indicator is used as KMnO4 itself acts as an internal indicator.
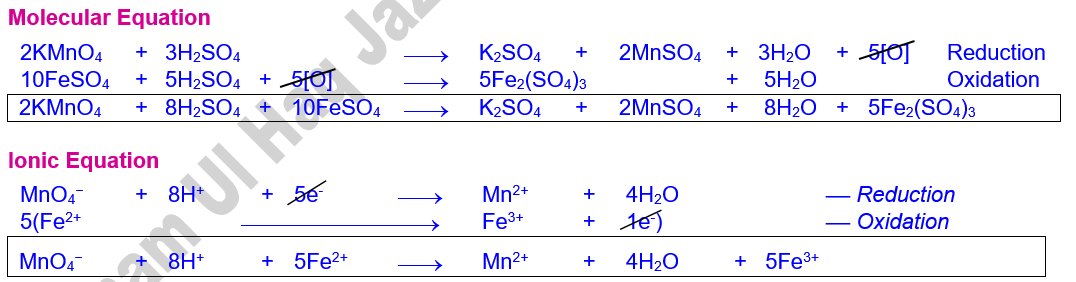
Molecular
Equation
Method
1. Wash the burette, pipette
and titration flask with water.
2. Clamp the burette
vertically in an iron stand and rinse the burette with Titrant (KMnO4)
solution. Now fill it with Titrant (KMnO4)
solution upto the zero mark.
3. After rinsing pipette with titrate (reducing agent) solution, pipette out 10 ml Titrate (reducing agent) solution into the conical flask and add equal volume of H2SO4 into it.
4. Run Titrant (KMnO4) solution from burette into flask drop-wise with constant shaking till a permanent pinkish tinge just appears by a single drop of Titrant (KMnO4). This is the end point. Note down the reading of the upper meniscus.
5. Now throw the content of
the flask and wash it with distilled water and repeat the above process of
titration till two similar readings (concordant readings) are obtained
Observations
1. Solution in the burette (Titrant) = KMnO4
2. Solution in conical flask (Titrate) = FeSO4 + H2SO4 (10 ml each)
3. Colour change = Colourless to pink
4. Normality of FeSO4.7H2O(N2) = ?
5. Volume of FeSO4.7H2O (V2) = 10 ml
6. Normality of KMnO4 (N1) = 0.1 N
7. Volume of KMnO4 (V1) = ?
8. Amount of FeSO4.7H2O in g/750 ml = ?
1. Normality
Determination
2. Amount Determination
Result
1. Normality of FeSO4.7H2O = _______ N
2. Amount of FeSO4.7H2O =
_______ g/750 ml
Redox Titration (Experiment No. 2)
Object
You are given 0.25 N solution of KMnO4. Find out the normality and amount of Mohr’s salt (ferrous ammonium sulphate) in gm/500 cm3.
Chemicals
1. Oxidizing agent –––– KMnO4
2. Reducing agent –––– Mohr’s
salt [FeSO4.(NH4)2SO4.6H2O].
3. Indicator –––– KMnO4 itself behaves as an indicator.
4. Helper / Medium –––– Acidic
(dilute H2SO4).
Theory
1. Titration is the type of quantitative volumetric analysis in which burette and pipette are used to standardize a solution. It is the process by which the strength of unknown solution is determined by treating it with a definite volume of a standard solution in the presence of an indicator.
2. Oxidation is the process during which oxidation number of an element is increased due to loss of one or more electron while reduction is the process during which oxidation number of an element is decreased due to gain of one or more electrons.
3.Oxidizing agent is a substance which gains electrons during reaction and thus they oxidize other substance and itself get reduced. e.g. KMnO4, K2Cr2O7 etc. Reducing agent is a substance which loses electrons during reaction and thus they reduce other substance and itself get oxidized. e.g. FeSO4.7H2O, FeSO4.(NH4)2SO4.6H2O, H2C2O4.2H2O etc
4. Both oxidation and reduction takes place simultaneously and such reactions are called Redox reactions.
5. The titration
between reducing agent and oxidizing agent in which one reactant is oxidized and
other is reduced is called Redox titration. In redox titration KMnO4
is always a Titrant (i.e. taken in the burette) while reducing agent is
titrate. The titration is carried out in acidic medium so dilute H2SO4
is also taken along with reducing agent in flask in equal amount. No external
indicator is used as KMnO4 itself acts as an internal indicator.
Method
1. Wash the burette, pipette and titration flask with water.
2. Clamp the burette vertically in an iron stand and rinse the burette with Titrant (KMnO4) solution. Now fill it with Titrant (KMnO4) solution upto the zero mark.
3. After rinsing pipette with titrate (reducing agent) solution, pipette out 10 ml Titrate (reducing agent) solution into the conical flask and add equal volume of H2SO4 into it.
4. Run Titrant (KMnO4) solution from burette into flask drop-wise with constant shaking till a permanent pinkish tinge just appears by a single drop of Titrant (KMnO4). This is the end point. Note down the reading of the upper meniscus.
5. Now throw the content of
the flask and wash it with distilled water and repeat the above process of
titration till two similar readings (concordant readings) are obtained
Observations
1. Solution in the burette (Titrant) =
KMnO4
2. Solution in conical flask (Titrate) = Mohr’s salt +
equal volume of dil. H2SO4
3. Colour change = Colourless to pink
4. Volume of KMnO4 (V1) = ?
5. Normality of KMnO4 solution (N1) = 0.25 N
6. Volume of Mohr’s salt (V2) = 10 ml
7. Normality of Mohr’s salt (N2) = ?
8. Amount of Mohr’s salt in g/500 cm3 = ?
Burette
Reading Chart
Calculations
1. Normality
Determination
2. Amount Determination
Result
1. Normality of Mohr’s salt = -------N
2. Amount of Mohr’s salt =--------g/500 cm3
Redox Titration (Experiment No. 3)
Object You are given 0.05 N solution of
hydrous oxalic acid (H2C2O4.2H2O). Find
out the
normality and amount of KMnO4 in g/950 cm3.
Chemicals
1. Oxidizing agent –––– KMnO4
2. Reducing agent –––– H2C2O4.2H2O
3. Indicator –––– KMnO4 itself acts as an indicator.
4. Helper / Medium –––– Acidic
(dilute H2SO4).
Theory
1. Titration is the type of quantitative volumetric analysis in which burette and pipette are used to standardize a solution. It is the process by which the strength of unknown solution is determined by treating it with a definite volume of a standard solution in the presence of an indicator.
2. Oxidation is the process during which oxidation number of an element is increased due to loss of one or more electron while reduction is the process during which oxidation number of an element is decreased due to gain of one or more electrons.
3. Oxidizing agent is a substance which gains electrons during reaction and thus they oxidize other substance and itself get reduced. e.g. KMnO4, K2Cr2O7 etc. Reducing agent is a substance which loses electrons during reaction and thus they reduce other substance and itself get oxidized. e.g. FeSO4.7H2O, FeSO4.(NH4)2SO4.6H2O,H2C2O4.2H2O etc
4. Both oxidation and reduction takes place simultaneously and such reactions are called Redox reactions.
5. The titration
between reducing agent and oxidizing agent in which one reactant is oxidized and
other is reduced is called Redox titration. In redox titration KMnO4
is always a Titrant (i.e. taken in the burette) while reducing agent is
titrate. The titration is carried out in acidic medium so dilute H2SO4
is also taken along with reducing agent in flask in equal amount. No external
indicator is used as KMnO4 itself acts as an internal indicator.
Method
1. Wash the burette, pipette
and titration flask with water.
2. Clamp the burette
vertically in an iron stand and rinse the burette with Titrant (KMnO4)
solution. Now fill it with Titrant (KMnO4)
solution upto the zero mark.
3. After rinsing pipette
with titrate (reducing agent) solution, pipette out 10 ml Titrate (reducing agent)
solution into the conical flask and add equal volume of H2SO4
into it.
4. Run Titrant (KMnO4)
solution from burette into flask drop-wise with constant shaking till a
permanent pinkish tinge just appears by a single drop of Titrant (KMnO4).
This is the end point. Note down the
reading of the upper meniscus.
5. Now throw the content of
the flask and wash it with distilled water and repeat the above process of
titration till two similar readings (concordant readings) are obtained
Observations
1. Solution in the burette (Titrant) =
KMnO4
2. Solution in conical flask (Titrate) = Oxalic acid +
Dilute H2SO4
3. Colour change = Colourless to pink
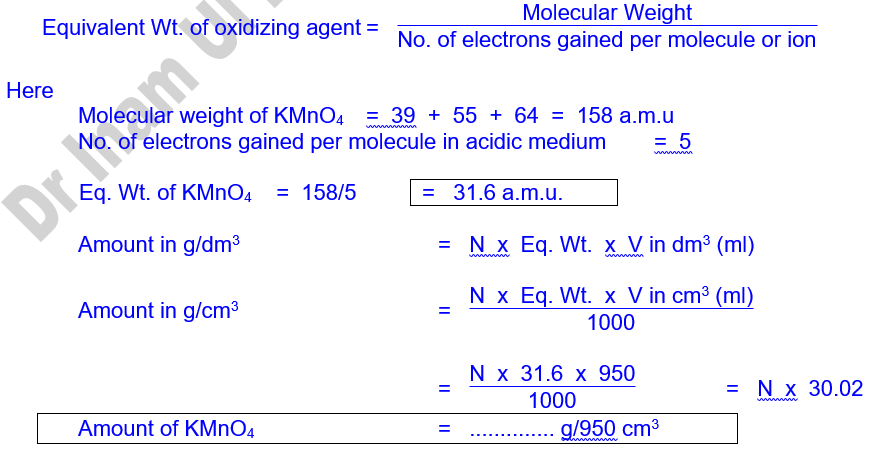
4. Normality of KMnO4 (N1) = ?
5. Volume of KMnO4 (V1) = ?
6. Amount of KMnO4 in gm/950 cm3 = ?
7. Normality of Oxalic acid (N2) = 0.05 N
8. Volume of Oxalic acid (V2) = 10 ml
Burette Reading Chart
Calculations
1. Normality Determination
2. Amount Determination
Result
1. Normality of KMnO4= _______ N
2.
Amount of KMnO4 =
_______ g/950 cm3
I. Element
Detection
Detection of Elements in the
Given Organic Compounds
4Na + O2 → 2Na2O ( Basic oxide)
2Na + 2H2O → 2NaOH + H2
Scheme of Element Detection
Model
Experiment No 1 (for Nitrogen)
Model Experiment No 2 (For nitrogen
and sulphur)
Model
Experiment No 3 (For Sulphur)
Model Experiment No 4 (For Chlorine)
Model Experiment No 5 (For Bromine)
IV. Determination of Boiling and Melting Points
IV. Determination
of Boiling and Melting Points
Determination of Boiling Point
Object
Determine the boiling point of given organic liquid.
Apparatus
Thermometer, ignition tube, thread, capillary tube, iron stand, tripod stand, wire gauze, Bunsen burner, glass rod, small beaker containing bath liquid (glycerin or paraffin oil
Theory
The temperature at which the vapour pressure of the liquid becomes equal to outside pressure (atmospheric pressure) is known as Boiling Point (B.P.).The normal boiling point is the temperature at which the vapour pressure of the liquid equals to one atmosphere (standard pressure).
Boiling point is the characteristic
constant of a liquid and it is the criteria of purity of liquids because pure
liquids always boil at a definite temperature showing sharp B.P.
Method (Capillary Tube Method / Siwoloboff’s Method
1. Take an ignition tube and put in it few drops of given liquid.
2. Attach the ignition tube with the bulb of a thermometer with the help of rubber band or thread.
3. Place a capillary tube whose upper end is sealed in the ignition tube.
4. Now hang the thermometer along with ignition tube in a beaker containing a liquid (water) of high boiling point (water bath).
5. Heat the beaker over wire gauze with constant stirring.
6. When a rapid stream of bubbles of vapours rises from the lower end of the capillary tube, then the temperature in thermometer is noted which indicates b.p. of given liquid.
7. Repeat the same process
to take at least three reading and find out concordant reading.
Observations

Result
The B.P. of given liquid is found out to be _____ °C.
Determination of Melting Point
Object
Determine the melting point of given compound.
Apparatus
Thermometer, thread, capillary tube,
iron stand, tripod stand, wire gauze, Bunsen burner, glass rod, small beaker
containing bath liquid (glycerin or paraffin oil)
Theory
The temperature at which there is an equilibrium between solid and liquid phases of a solid is called melting point. OR Melting point is the temperature at which both liquid and solid phases coexist at equilibrium.
Melting point is the characteristic
constant of a solid and it is the criteria of purity of solids because pure
solids always melt at a definite temperature showing sharp M.P.
Method
1. Take a capillary tube and seal its lower end by heating.
2. Put a little quantity of powdered compound in a capillary tube.
3. The capillary tube is then fixed to a bulb of thermometer with the help of rubber band or a thread.
4. Now thermometer with capillary tube is suspended in water bath (beaker containing bath liquids like glycerin or water) by means of iron stand in such a way that upper end of capillary tube should be kept out of the liquid.
5. Heat the beaker with constant stirring till solid given compound shows signs of melting and then remove the burner.
6. Stir continuously till
wax becomes transparent. Note this temperature at which compound just becomes
transparent. This will be m.p. of given compound.
Observation
Result
M.P. of the given compound is found out to be _____ °C.
VI. Comprehensive Question Answer
Viva-Voce
Simple and Redox Titrations
Q1. What is the volume of burette?
Ans: 50.0cm3
Q2. Name two organic acids which are diprotic?
Ans: Oxalic acid, succinic acid
Q3. Indicate
the amounts in grams required to prepare 1N and 1M one liter of oxalic
acid solution.
Ans: 63.0 g for 1N solution, 126.0 g for 1M for
solution.
Q4. Name any two hydrates used in simple
titration.
Ans: Oxalic acid (H2C2O4.2H2O),
Washing soda (Na2CO3.10H2O)
Q5. What is primary standard solution?
Ans: It is a substance which is 100 percent pure
or of a known purity.
Q6. Why NaOH and KOH are not primary standard?
Ans: Because they absorb water (moisture) and CO2
from air.
Q7. Is anhydrous sodium carbonate a primary
standard?
Ans: Yes, it is.
Q8. What is pH
range of phenolphthalein? OR Why is phenolphthalein taken as an indicator when weak acid is titrated against
strong base?
Ans: The pH range of
phenolphthalein is 8.3 to 10.0.The basic pH range of phenolphthalein
makes it a suitable indicator when
weak acid is titrated against strong base as it produces sharp colour change at pH round about 8 to 10.
Q9. What is pH
range of methyl orange? OR Why is methyl orange taken as an indicator when
strong acid is titrated against weak base?
Ans: The pH range of
methyl orange is 2.9 to 4.6.The acidic pH range of methyl orange makes it a suitable
indicator when strong acid is titrated against weak base as it produces sharp colour change at pH round about 3 to 5.
Q10. Is KMnO4 a primary standard solution?
Ans:No, as KMnO4 can not be obtained
in pure form and its solution in not very stable.
Q11. Name two coloured salts of potassium.
Ans; i.Potassium permanganate (KMnO4) ii. Potassium dichromate (K2Cr2O7)
Q12. Name two coloured salts of sodium.
Ans: i.Sodium chromate (Na2CrO4) ii. Sodium dichromate (Na2Cr2O7)
Q13. Give 3 reasons responsible for formation of
brown precipitates in redox titration.
Ans; The formation of brown ppt in KMnO4
titration is due to:
i.
The solution may be very
cold.
ii.
KMnO4 might have
been added in large amount and too quickly.
iii.
The quantity of dilute H2SO4
added may be insufficient.
Q14. Why does phenolphthalein remain colourless
in acidic medium and give pink colour in basic
medium?
Ans: Phenolphthalein is a weak organic acid. Its
unionized molecule is colourless and its
anion is pink coloured. It is
feebly ionized to give H+ and pink coloured anion (In-). Under acidic conditions, its ionization equilibrium is
forced to left due to common ion effect thereby lowering the concentration of its pink coloured anion (In-), so solution becomes
colourless. Under alkaline conditions,
the added OH¯ ions combine
with its H+ ions to form feebly ionized water molecule thereby increasing the ionization of
HIn (phenolphthalein) giving more and more pink coloured anion In¯ and thus solution turns
pink.
![]()
Q15. Why does methyl orange give reddish pink in
acidic medium and faint yellow colour in basic
medium?
Ans: Methyl orange is a weak organic base. Its
unionized molecule is yellow and its cation is reddish pink
coloured.It is feebly ionized to give OH¯ ions and reddish pink
coloured cation (Me+). Under basic
conditions, its ionization equilibrium is pushed to left due to common ion
effect thereby lowering the concentration
of its pink coloured cation and increasing the concentration of its yellow coloured unionized molecule, so
solution turns yellow. Under acidic conditions ,the added H+ ions combine with its OH¯ ions to form feebly ionized
water molecule thereby increasing
the ionization of MeOH (methyl orange),
giving more and more reddish pink coloured cation Me+ and thus solution turns yellow to pink.
![]()
Q16. What is meniscus?
Ans: The surface of a liquid in cylindrical
vessel is called meniscus means moon.
Q17. Which meniscus is taken for burette reading
of coloured solution?
Ans: Upper meniscus.
Q18. Which meniscus is taken for burette reading
of colourless solution?
Ans: Lower meniscus.
Q19. Why do
we note upper meniscus of KMnO4 solutions?
Ans: KMnO4 is highly coloured compound.
Its lower meniscus is not adequately observed or distinctly visible due to its intense colour
and hence upper meniscus is recorded.
Q20. Why KMnO4 solution is kept in
coloured bottles?
Ans: KMnO4 solution decomposes on
exposure to light and air, hence it is kept in dark coloured bottles.
Q21. How will you store a standard stock solution
of KMnO4?
Ans: It is stored in dark or amber-coloured
bottles and should be protected from light
Q22. Can HCl be used instead of dilute H2SO4
in redox titration?
Ans: HCl cannot be used in redox titration as
some of KMnO4 may be consumed for oxidizing HCl into chlorine.
Q23. Why KMnO4 is always acidified with
H2SO4 and not with HCl or HNO3?
Ans: KMnO4 is always acidified with H2SO4
because sulphuric acid is not self oxidizing and also it has no action upon KMnO4 in
dilute solution.KMnO4 can never be acidified with HCl or HNO3 as
nitric acid is itself an oxidizing
agent and HCl itself oxidized by KMnO4 into chlorine gas.
2MnO4− +
16H+ + 10Cl− ¾® 2Mn2+
+ 8H2O + 5Cl2
Q24. In Mohr’s salt which is oxidized and which
remain unaffected?
Ans: Ferrous sulphate is oxidized to ferric
sulphate while ammonium sulphate remains unaffected during titration.
Q25. Is heating necessary for titration of KMnO4
with ferrous sulphate?
Ans: No, because on heating ferrous sulphate is
oxidized to ferric sulphate. Hence if we heat ferrous sulphate the titration can not be performed accurately.
Q26. Why oxalic acid solution is heated before
titrations?
Ans: The reaction between KMnO4 and
cold oxalic acid solution is very slow due to slow production of Mn2+ ions. In order to
speed up the reaction and for rapid production of Mn2+ ions, to
complete the reaction, heating
is done.
Q27. What happens to oxalic acid in redox
titration in the presence of dilute H2SO4?
Ans: Oxalic acid is oxidized to CO2 by
oxidizing agent KMnO4.
Q28. Why we do not add any other indicator in
KMnO4 titration? Or Why
KMnO4 is called self-indicator?
Ans: Because KMnO4 is a
self-indicator and highly coloured due to presence of permanganate ion which is pink in colour .When it
reacts a reducing agent solutions during titration, a very light pink-coloured product, manganous sulphate
(seem to be colourless) is obtained. When the reaction
is over, even one drop of KMnO4 produces pink colour.
Q29. What is the function of KMnO4 in
redox titration?
Ans: KMnO4 acts as an oxidizing agent
and provides nascent oxygen or gains electrons (3e- in basic medium and 5e- in acidic medium).It also
acts as a self-indicator or internal indicator.
Q30. What is the function of H2SO4
in redox titration?
Ans: It helps to increase the oxidizing tendency
of KMnO4. In the presence of sulphuric acid, KMnO4
becomes strong oxidizing agent
and extract 5 electrons from reducing agent (which is being oxidized) and gets reduced to
manganous (Mn2+) ion.
|
MnO4− |
+ |
8H+ |
+ |
5e− |
¾® |
Mn2+ |
+ |
4H2O |
|
Q31. Why a burette with glass tap is used in
redox titrations?
Ans: Rubber tap should not be used as KMnO4
being an oxidant acts powerfully on rubber. Thus burette with glass tap
is used in redox titrations.
Q32 What are the sources of following acids?
Citric acid, Tartaric acid, Acetic acid,
Lactic acid, formic acid
|
Ans: (i) |
Citric
acid |
––––– |
Lemon |
|
(ii) |
Tartaric
acid |
––––– |
Grapes |
|
(iii) |
Acetic
acid |
––––– |
Vinegar |
|
(iv) |
Lactic
acid |
––––– |
Fermented milk |
|
(v) |
formic acid |
––––– |
Stings of
bees, wasps |
Q33. What is
Oxonium/Hydronium/Hydroxonium Ion (H3O+)?
Ans: The solvated
(hydrated) proton i.e. a proton (H+) which accepts a lone pair of
electron from water is known as
Oxonium or Hydroxonium Ion (H3O+).
![]()
![]()
Q34. What is Degree of Dissociation?
Ans: The ratio of the number of molecules ionized to the total number
of dissolved molecules of a substance
is known as Degree of Dissociation (a) i.e.
Q35. What are the
precautions taken while performing simple titration?
Ans: Following precautions
must be taken while performing simple titration:
(i)
Burette must be clamped
vertically.
(ii)
Burette should be filled with
the help of funnel.
(iii)
Lower meniscus of liquid in
the burette should be noted.
(iv)
Air bubbles must be removed
from the jet of the burette before starting experiment.
(v) Rinse the burette
and the pipette with NaOH and HCl respectively but conical flask must be washed
with water and never rinse with acid.
Q36. Why alkali is taken in
burette when phenolphthalein is used as an indicator? Or Why phenolphthalein is
preferred as indicator when base is taken in burette?
Ans: Phenolphthalein is a
suitable indicator when colour change is required at basic pH round about 8 to 10 which it gives by producing sudden
sharp colour change when strong base is used. When strong base is used it is always taken in burette as the
appearance of pink colour at the end point is
distinctly visible
Q37. What are hydrates or hydrous compounds?
Ans: The compounds containing water of
crystallization as an essential part of their crystals are called hydrates or hydrous compounds.
Q38. What is meant by water of crystallization?
Ans: The constant number of water molecules attached
to the ions (mostly cations) of ionic salts (hydrates)
is called water of crystallization.
Q39. What is meant by anhydrous salts?
Ans: The hydrous salts without water of
crystallization are called anhydrous salts.
Q40. What are hygroscopic substances?
Ans: Hygroscopic substances absorb moisture on
exposure to the atmosphere but not form solution and merely get moist or sticky.
Q41. Give some examples of hygroscopic
substances?
Ans: NaNO3, CuO,
CaO
Q42. What is efflorescence and efflorescent?
Ans: The phenomenon of losing part or all water
of crystallization by some crystalline salts on exposure to
the atmosphere to form a lower hydrate or anhydrous salt is called
efflorescence and the salt is said
to be efflorescent.
Q43. Give example of efflorescent.
Ans: Na2CO3. 10H2O
(which loses 9 out of its 10 molecules of water of crystallization).
Q44. What is meant by deliquescence and
deliquescent?
Ans: Some
compounds tend to absorb a large amount of water on exposure to the atmosphere
thereby turning into solutions. This phenomenon is known as Deliquescence and
the substances are said to be Deliquescent.
Q45. Give examples of some deliquescent.
Ans: NaOH, KOH,
FeCl3, MgCl2, CaCl2, P4O10.
Q46. On which factor hydration ability of ions
depend?
Ans: Hydration ability of ion depends on its
charge density. Greater the charge density of ion, greater will
be its hydration ability and vice-versa.
Q47. Give name and formulae different hydrates
used in theory and practical.
Ans:oxalic
acid, sodium carbonate, ferrous sulphate, Mohr’s salt, blue vitriol
Q48. What is hydrated ion?
Ans: The ion surrounded by water molecules is
called hydrated ion.
Q49. What are strong acids and bases?
Ans: Acids or bases which
completely ionize having high %age dissociation (usually 30 % or more) in
aqueous solution are considered to be strong. In terms of ionization constant
strong acids and bases are those which have large value of ionization constant
usually greater than 1
Q50. What are weak acids and bases?
Ans: Acids or bases which
partially ionize having low %age dissociation (usually below 30 %) in aqueous
solution are considered to be weak. In terms of ionization constant weak acid
and bases are those which have small value of ionization constant usually less
than 1
Element Detection
Q51. What are organic compounds?
Ans: The covalent compounds of carbon with other
elements such as H, O, S N, P, and halogens are
called organic
compounds with the exception of carbonates, bicarbonates, cyanides, sulphocyanides,
thiocynates, carbides and oxides of carbon.
Q52. Which element is regarded as the chief
constituent of all organic compounds?
Ans: Carbon is considered to be an essential
element of all organic compounds.
Q53. What are the other elements that are
generally present in the organic compounds?
Ans: Organic compound contain mostly hydrogen
and oxygen. Beside these they may contain elements
like nitrogen, sulphur, chlorine, bromine, iodine and some metallic
elements like sodium, magnesium,
calcium, copper, lead, silver, iron, lithium, potassium etc. Usually the atomic
number of those elements range
form 1 to 20 except Br and I and some metals.
Q54. Which type of bonding is present in organic
compounds?
Ans The bonding in organic compounds is
essentially covalent in the form of single, double, triple, polar or nonpolar bonds.
Q55. What type of reactions take place in organic
compounds?
Ans: Organic compound undergo molecular or
nonionic reactions. These reactions include substitution reaction, addition reactions,
elimination reactions, oxidation reactions etc.
Q56. Can we detect an element directly in organic
compound? Or Why are the organic compounds
fused with sodium metal?
Ans: organic
compounds are non-ionic or covalent in nature and do not dissociate into ions
when put in water. In
addition, due to covalent bonding they do not undergo ionic reactions instead
give molecular reactions which
are very slow. Hence detection of different elements present in organic compounds directly becomes
very difficult by ordinary reactions. Hence they are converted to water-soluble ionic sodium salts by fusing with sodium
metal.
Q57. Why is sodium extract prepared?
Ans: Sodium extract is prepared to change the
nature of bonding in organic compounds from covalent to ionic.
Q58. What happen when sodium extract is prepared?
Ans: The organic compounds are decomposed and
the elements present in them form ionic water soluble
salts with sodium.
Q59. How does the element present in organic
compounds change into sodium salt?
Ans: When an organic compound containing nitrogen,
sulphur or halogens is fuses with sodium, the nitrogen
of the organic compound changes to water-soluble sodium cyanide, sulphur
changes to sodium sulphide and
halogens are converted into respective sodium halides. If both nitrogen and sulphur are present, they are converted into sodium sulphocynides.
Q60. How are the elements detected from the
sodium extract?
Ans: The elements are detected as radicals by
means of qualitative analysis. The presence of CN¯, S2-, Cl¯, Br- and I¯ radicals confirm
the presence of nitrogen, sulphur, chlorine, bromine and iodine respectively
in the given organic compounds.
Q61. What is Lassaign’s filtrate or Sodium
Extract?
Ans: Sodium Extract or Lassaign’s Filtrate is
Sodium Salt containing elements as ions. It is prepared by fusing organic compounds with sodium metal followed by dissolution
in water.
Q62. Why sodium metal is preferably taken to make
Sodium Extract?
Ans: It is cheap and soft,
so easy to cut into pieces. It melts at low temperature (98°C).
It is very
reactive metal and due to its high chemical reactivity it reacts readily with other
elements. In addition, all sodium salts
are water soluble and can undergo simple ionic reactions.
Q63. Why sodium metal is always kept in kerosene
oil?
Ans: Sodium is very reactive metal owing to its
prior position in the reactivity series. On exposure to atmosphere it reacts with air and moisture to form its respective
oxide and then hydroxide readily.
Thus it is immersed in liquid containing
no oxygen to prevent its reaction with air.
|
|
Q64. What is the nature of sodium
extract?
Ans: It is mostly alkaline because of the oxidation
of unreacted sodium to basic sodium oxide which upon dissolving in water yield sodium hydroxide. In addition unreacted
sodium directly reacts with water to
form sodium hydroxide.
|
|
||||
Q65. Why is concentrated sulphuric acid added in
the test for nitrogen?
Ans: Conc. H2SO4 is added
in N-Test because first FeSO4 is converted into green ppt. of ferrous
hydroxide, Fe(OH)2
by combining with NaOH formed during the preparation of Na-Extract and these ppt will mask the green or blue colour
of nitrogen test and will mislead our
result. The green ppt of ferrous
hydroxide is dissolved in conc. H2SO4 leaving Prussian
blue colour which is a hallmark of nitrogen
test.
Q66. We only add the ferrous sulphate, from where
the ferric ions come?
Ans: Although we do not provide ferric ions but
they already formed by the oxidation of ferrous sulphate upon heating.
Q67. Is it necessary to add ferric chloride in
the test for nitrogen?
Ans: No, it is not
compulsory to add ferric chloride in the test for nitrogen. The purpose of
addition of ferric chloride is to supply ferric ion, which may already be
produced by the oxidation of ferrous ion upon boiling.
Q68. Can concentrated HCl be used instead of H2SO4
in the test of nitrogen?
Ans: HCl can be used in nitrogen test as it also
dissolves green ppt of ferrous hydroxide. However it is better to use sulphuric acid because if HCl is used the colour
of the solution turns yellow due to the
formation of ferric chloride,FeCl3 instead of Prussian blue which
may mislead our result.
Q69. Why do we get Prussian blue colour if
nitrogen is present?
Ans: The Prussian blue colour is due to the
formation of a complex called ferric ferrocyanide with formula Fe4[Fe(CN)6)]3
Q70. Why acetic acid is added before lead acetate
in the test for sulphur?
Ans: Acetic acid is added to neutralize NaOH
present in alkaline sodium extract which (NaOH) would otherwise react with lead acetate to form white ppt of
lead hydroxide.
|
|
2NaOH +
Pb(CH3COO)2 |
¾¾¾¾® |
Pb(OH)2¯ |
+ 2CH3COONa |
|
|
|
|
White ppt |
|
Q71. Why a black ppt appear in the test for
sulphur?
Ans: It is because of the
formation of black coloured insoluble lead sulphide (PbS). It is noteworthy that all
sulphides are black with the exception of CdS, SnS2 and Sb2S3.
Q72. Why does violet colour appear in the
confirmatory test for sulphur?
Ans: The violet colour appears in the C.T. for
sulphur due to formation of violet coloured complexcommonly called Sodium sulphoprusside or Sodium thionitroprusside.
Its IUPAC name is sodium pentacyanothionitroferrate(II)
with formula, Na4[Fe(CN)5NOS]
Q73. Why dilute nitric acid is added before
adding silver nitrate in the halogen test?
Ans: Dilute nitric acid is added to neutralize
NaOH present in alkaline sodium extract which (NaOH) would otherwise
react with silver nitrate to form brown ppt of silver hydroxide or silver
oxide. When Na-Extract
containing Cl¯ion is boiled with HNO3 (dil), the
remaining traces of CN¯ and S2- ions are removed. The presence of CN¯
and S2¯ ions in Na-Extract gives white ppt. of AgCN or Black ppt. of Ag2S with AgNO3
|
|
NaOH |
+ |
AgNO3 |
¾¾¾¾® |
AgOH¯ |
+ |
NaNO3 |
|
|
|
|
|
|
Brown ppt |
|
|
|
|
|
|
2AgOH |
¾¾¾¾® |
Ag2O |
+ |
H2O |
Q74. How do you differentiate the presence of chlorine,
bromine and iodine in halogen test?
Ans: In halogens test, the ppt of different
colours and their reaction with ammonia to form complex differentiate chlorine, bromine and iodine
i. Chlorine is indicated by the formation of white ppt. of AgCl (with AgNO3) which are soluble in excess of NH4OH due to formation of soluble Silver-Ammonia Complex called diamminesilver(I) Chloride.
ii. Bromine is indicated by the formation of pale yellow ppt. of AgBr (with AgNO3) which are partially soluble in excess of NH4OH due to formation of partially soluble Silver-Ammonia Complex called diamminesilver(I) bromide.
iii. Iodine is indicated by the formation of deep yellow ppt. of AgI (with AgNO3) which are insoluble in excess of NH4OH
Q75. What is layer test?
Ans Layer Test is a C.T. for Bromine and
Iodine. Layer Test is based upon Displacement Reaction.
Q76. What is the principal or basis of layer
test?
Ans: The Layer Test is based upon the fact that
a more electronegative element (i.e. Cl2) displaces a weak electronegative element or ions (i.e. Br- or I-) from its salts.
The displaced ions evolve in the
form of coloured vapours which dissolve in CCl4/CHCl3 to
form coloured layer at the bottom.
Q77.What is the function of chlorine water in layer test?
Ans: The Chlorine water acts as an oxidizing
(displacing) agent and oxidizes (displaces) Br¯ ion or iodide (I¯) ion from its salt (NaBr or NaI) to Br2
vapours or I2 vapours.
Q78. What is the function of chloroform or carbon
tetrachloride in the layer test?
Ans: The function of chloroform (CHCl3)
or carbon tetrachloride (CCl4) is to dissolve Br2 vapours
or I2 vapours displaced by
Cl2–water thereby giving brown or violet coloured layer bottom or
top.
Q79. Can any other liquid be used instead of carbon
tetrachloride in layer test?
Ans: Yes, any non-oxy organic solvent can be
used like benzene, chloroform in layer test. However benzene and chloroform will form upper layer as they are
lighter than water while carbon tetrachloride
forms lower layer as it is heavier than water.
Boiling and melting
point
Q80. What is boiling?
Ans: Boiling is the process of rapid conversion of liquid into
vapours at specified temperature (i.e. at its boiling
point) when vapour pressure of the liquid becomes equal to atmospheric pressure
that takes place throughout the mass
of liquid.
Q81. What is evaporation?
Ans: Evaporation is the slow conversion of liquid to gaseous state at
room or all temperatures that takes
place only at the liquid’s surface.
Q82. What is
boiling point (b.p.)?
Ans: The temperature at
which vapour pressure of a liquid becomes equal to outside atmospheric pressure (which is normally 760 torr) is
called boiling point.
Q83. What is normal boiling point (b.p.)?
Ans: Normal boiling point
is the temperature at which vapour pressure of a liquid becomes equal to standard atmospheric pressure or 760 torr
is called boiling point.
Q84. What is the significance of boiling point?
Ans: Boiling point is the criteria of purity of liquid because a pure
liquid boils at a definite temperature. Thus b.p. is a characteristic constant of
a liquid which can be used for testing the purity of a liquid.
Q85. What is the effect of pressure on b.p?
Ans: Boiling point
increases with the rise of pressure and is lowered with the decrease in pressure
i.e. b.p. is directly proportional to pressure.
Q86. What is the effect of height on b.p?
Ans: With
increase in altitude (height), b.p. is reduced as the pressure decreases. That is
why cooking food is difficult and takes longer time at high altitude mountain
where pressure is below normal i.e. below 760 torr.
Q87. What is the effect of impurities on b.p?
Ans: Volatile impurities elevate the b.p. of a liquid.
Q88. What is water bath?
Ans: The beaker containing a non-volatile liquid (e.g. water) which
is used for heating the volatile liquids
indirectly is known as water bath.
89. Name some bath liquids
Ans:(i) Paraffin oil (ii)Concentrated sulphuric acid (iii) Glycerine
Q90. What is vapour pressure?
Ans:The pressure exerted
by vapours in equilibrium with its pure liquid at a particular temperature is called vapour pressure or equilibrium
pressure.
Q91. What is the effect of temperature on vapour pressure?
Ans: Vapour pressure increases with the rise of temperature and vice
versa.
Q92. What is latent heat of vaporization?
Ans: The amount of heat energy required to vapourize one gram of a
liquid at its b.p. without change in temperature
is called Latent Heat of Vaporization.
Q93. Why at b.p.,
the temperature remains constant inspite of continuous supply of heat?
Ans: It is because at b.p,
molecules of liquid attain maximum average kinetic energy and further heat provided at b.p. instead of raising the
temperature of the liquid is used to overcome intermolecular
attractive forces and is utilized for converting liquid into vapours. This
heat is called Latent Heat of
Vaporization. Thus average K.E. of liquid molecules or temperature of liquid at b.p. remains constant at its maximum.
Q94. What are the
precautions taken while noting boiling point?
Ans: Following precautions are taken while noting down boiling point:
(i) The
capillary tube should be long enough.
(ii) The water bath should be heated slowly
with constant stirring for uniform distribution of temperature.
(iii) Reading of thermometer should be noted
when steady stream of bubbles from the lower end
of capillary tube begin to arise continuously.
Q95. What is
melting or fusion?
Ans: Melting or fusion is the characteristic of solid by virtue of
which it is converted into liquid on heating
at its melting point.
Q96. What is
melting point (m.p.)?
Ans: Melting point is the
temperature at which both liquid and solid phases co-exist at equilibrium. OR It
is the temperature at which there is an equilibrium between solid and liquid
phases of a solid.
Q97. What is the significance of melting point?
Ans: Melting point is the criteria of purity of solids as pure
substances have sharp m.p.
Q98. What is effect of impurity on m.p?
Ans: Impurities (volatile)
lower the m.p.
Q99. What is the effect of pressure on m.p. of solid which expands on
freezing?
Ans: The substances which expand on freezing, have a fall in m.p with
the increase in pressure i.e. m.p.
decreases with pressure.
Q100.What is the effect of
pressure on m.p. of solid which contracts on freezing?
Ans: The substances which contract on freezing, have a rise in m.p.
with the increase in pressure i.e. m.p.
increases with pressure.