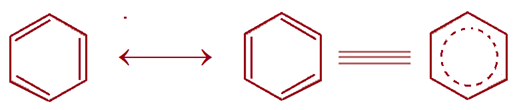
Kekule’s Structure (Formula) of Benzene
Straight Chain Proposed Structures
For many years, there was speculation over the structure of benzene and the structure and bonding in benzene ring was debated for about 30 years. After 1834, when molecular formula as established as C6H6, scientists put forward hexatetraene unsaturated linear open chain formula such as H2C=C=CH–CH=C=CH2 (as from the formula C6H6, one might expect it to be a highly unsaturated compound). But in fact, benzene appeared to be strangely unreactive in comparison with the alkenes, undergoing substitution reactions rather than the expected addition reactions of unsaturated compounds.
1. Hexadiyne (2 structures)2. Hexadienyne (1 structure)
3. Hexatetraene (1 structure)
Straight Chain Structure is ruled out

Ruling out of Straight Chain Structures
1. Benzene does not give addition reaction with KMnO4 as alkene or alkynes.
2. Benzene does not follow general formula of alkane (CnH2n+2), alkenes (CnH2n) and alkynes (CnH2n-2).
3. Straight chain compound of six carbon chain gives 3 substituted products whereas benzene gives only one mono-substituted product.
4. Benzene prefers to give substitution reaction rather than addition reactions. Benzene can add 3 molecules of various reagents like H2 or Cl2 or O3 which suggests the presence of 3 pi bonds in its structure.
CYCLIC REGULAR HEXAGONAL HEXATRIENE RING STRUCTURE for BENZENE
In 1865 a German chemist, Kekule suggested a CYCLIC REGULAR HEXAGONAL HEXATRIENE RING STRUCTURE with alternate three C – C double bonds for benzene to explain its unusual behaviour, why benzene gives only one monosubstituted product, three disubstituted products and adds 3 moles of hydrogen, halogens or ozone. No linear formula would correctly account for the number of substituted products but the cyclic structure solved the problem. According to Kekule’s formula, benzene should undergo addition reactions.
Evidences in Support of Kekule’s Structure
2. Formation of only one monosubstituted product of benzene
3. Formation of three isomeric disubstituted products of benzene
4. Confirmation from X-rays Analysis
1. Addition of 3 molecules of H2 or X2 to give Saturated Cyclic Compound
Under rigorous conditions, benzene undergoes addition reactions like hydrogenation and halogenation, adding three molecules of hydrogen in the presence of Pt and three molecules of chlorine or bromine in sunlight under high pressure to convert into saturated alicyclic compounds namely cyclohexane and hexachlorocyclohexane (or hexabromocyclohexane) respectively. These addition reactions indicate the presence of three double bonds in a benzene molecule.
There are two possible explanations for behaviour:
(i) Firstly, only one of the six hydrogen atoms is reactive.
(ii) Secondly, all the six hydrogen atoms are identical and equivalent and replacing any of them results in the same monosubstituted product.
Hence, benzene possesses a regular hexagonal ring having double bonds alternate with single bond undergoing substitution reactions in which its cyclic structure is preserved.
3. Formation of Three Isomeric Disubstituted Products of Benzene
Monosubstituted benzenes (MSB) on disubstitution yield three isomeric disubstituted products of benzene i.e. ortho (2,6), meta (3,5) and para (1,4) isomers. This can be explained from the Kekule’s structure. Since benzene possess symmetrical hexagonal ring, the position 2 and 6, 3 and 5 and 1 and 4 are identical. For this reason, there are three different disubstituted products possible.
For example; Xylene (a DSB) has three isomeric forms.
4. Confirmation from X-rays Analysis
The X-rays analysis gives the following shape to benzene molecule which shows:
Each bond angle (C-C-H & C-C-C) = 120°
Each C–C bond length = 1.397°A/1.34°A (intermediate b/w 146 pm and 134 pm)
Each C–H bond length = 1.09°A (1.08°A as in alkene)
Objections in Kekule’s Structure
Kekule’s structure favours the unsaturation of benzene while benzene acts as a saturated hydrocarbon in most of its reactions undergoing substitution reaction. Benzene is stable compound.
1. Unusual and strange character of double bond in benzene ring.
2. Another objection to Kekule formula was that benzene would give two distinguishable disubstituted orthoisomers when substitution occurs at position 1 and 2 whereas in practice only was isolated. e.g. ortho xylene would exist in two isomeric forms, one of the isomer has both the substituents (methyl groups) on carbons containing a single bond while the other isomer has both the substituents on carbons having a double bond [i.e. in isomer I, there is a single bond and in isomer II, there is a double bond between two substituents (methyl groups)]. Such isomerism has never been observed in practice.

Dynamic Formula / Resonance Formula (1872)
To account for why only one product is obtained, Kekule suggested that double bonds in benzene are not stationary or fixed but in a state vibration flipping back and forth, giving rise to a single bond between two adjacent carbons at one instance and a double bond at the other instance. This results in the oscillation of double bonds indicating that all C–C bonds have a partial double bond character.
[This explanation shows the existence of both types of molecules at any instance. As the two forms I and II are in rapid equilibrium then separation is therefore not possible].
Defects in Dynamic Kekule’s Formulae
1. X-rays analysis showed that all the C–C bonds in benzene have the same bond lengths of 140 pm (or 0.1397 nm or 1.397°A) instead of 134 pm ( or 0.134 nm or 1.34°A) for a double bond and 146 pm (or 0.146 nm or 1.46°A ) for a C–C single bond and all the bond angles (C–C–C and C–C–H) in benzene have the same angle of 120° both of which correspond to perfect sp2-hybridization. Interestingly, the 140pm bond distances in benzene are exactly midway or intermediate between the typical sp2-sp2 C-C single bond length of 146 pm and the sp2-sp2 double bond distance of 134 pm. If bond distances are related to the bond type, what kind of carbon-carbon bond is it that lies halfway between a single bond and a double bond in length!!!

2. Calculated chemical potential energy for Kekule’s structure is 1264 kcal/mole while it is experimentally found as 1300 kcal/mole. This difference of energy of 36 kcal/mole (150 kJ/mole) is in fact the resonance or stabilization energy of benzene. Thus benzene is more stable than represented by Kekule’s formula.

Conclusion
Kekule’s structure is not a true representation of benzene. Actual structure is a resonance hybrid of two Kekule’s structures.