MCQs on Chapter # 4 …… Chemical Bonding
1. An example of ionic compound is:
(a) H2
(b) CH4
(c) NaCl
(d) N2
2. Interaction between highly electron deficient hydrogen and highly electronegative atom is called
(a) ionic bond
(b) metallic bond
(c) hydrogen bond
(d) covalent bond
3. Two fluorine atoms share 1 electron each in their outermost shell to achieve electronic configuration of:
(a) Xe
(b) Ar
(c) Kr
(d) Ne
4. Number of electrons lost by atoms of group IIIA equals:
(a) 1
(b) 2
(c) 3
(d) 4
5. Atom which loses two electrons from its outer shell to form ion is called:
(a) oxygen
(b) potassium
(c) magnesium
(d) carbon
6. In NaCl crystal lattice each Na ion is surrounded by:
(a) 6 Cl‒ ions
(b) 6 Na+ ions
(c) 8 Cl‒ ions
(d) 12 Cl‒ ions
7. At room temperature most of ionic compounds are
(a) amorphous solids
(b) crystalline solids
(c) liquids
(d) gases
8. Tendency of atoms to acquire eight electrons in their valence shell is:
(a) octet rule
(b) duplet rule
(c) triplet rule
(d) none of them
9. When one atom forms cation by losing electron and other forms anion by accepting that electron then bond form between them is
(a) coordinate covalent bond
(b) Covalent bond
(c) Ionic bond
(d) hydrogen bond
10. Noble gases are stable because they contain ………. electrons in valence shell
(a) 4
(b) 6
(c) 8
(d) 10
11. Bond which
involve 3 shared electron pairs is a :
(a) double covalent bond (b) single covalent bond (c) triple covalent bond (d) none of above
12. A non-metal
atom form anion by
(a) loses of electrons (b) gain of electrons (c) loses of protons (d) gain of protons
13. When two
identical atoms share electron pairs and exert force on each other than bond
form is:
(a) coordinate covalent bond (b) non-polar covalent bond (c) double covalent bond (d) polar covalent bond
14. Synthetic
resins are used on places where:
(a) electric resistance is required (b) water resistance is required (c) adhesion is required (d) friction is required
15. Oxygen belongs
to group VIA so number of electrons in its valence shell are:
(a) 3 (b) 4 (c) 5 (d) 6
16. Electron pairs
which are not shared by atoms are called:
(a) Bond pair (b) lone pairs (c) shared pairs (d) electron pairs
17. Strength of
intermolecular forces from ionic or covalent bond is:
(a) Weaker (b) stronger (c) equal (d) none of above
18. Ionic crystals
have:
(a) high melting points (b) moderate melting points (c) low melting points (d) none of above
19. Bond formed by
mutual sharing of electron is:
(a) coordinate covalent bond (b) covalent bond (c) metallic bond (d) ionic bond
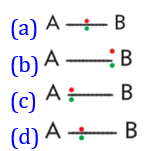
20. Which of the
following diagram shows atoms are bonded with same electronegativity?