Isomerism and Its Types
Definition,
Basis and Explanation
The word isomer is a combination of two words, iso means same and mers means unit and
this term was invented by Berzelius. The compounds which differ in their properties but
have same molecular formula are called isomers and the phenomenon is known as
isomerism.
Isomerism is the existence of
different compounds exhibiting different physical or chemical properties or
both having same molecular formula i.e. The phenomenon of existence of two or more compounds possessing
the same molecular formula but different properties is known as isomerism. Such
compounds are called as isomers.
Isomers refer to those compounds which have the same molecular
formula but differ in physical or chemical properties or both i.e. Isomers have entirely different physical properties and in many
cases also have distantly different chemical properties (except chain isomers,
metamers).
Isomers refer to two or more compounds having
Same MF, same number of atoms, same nature of atoms, same EF,
same degree of unsaturation, same MW, same vapour density, same number of bonds
but differ at least in one physical or chemical properties.
Reason of Isomerism
Isomerism is due to the different arrangement of atoms or groups in a molecule (structural isomerism) or due to different spatial configuration of the atoms or groups (stereoisomerism).
Importance
Isomerism is possible for compounds containing at least four carbon
atoms. Number of isomers increases with increase in number of carbon atoms in
saturated hydrocarbons.
Example # 1
The concept of isomerism can be illustrated by referring to two different
compounds, nitromethane and methyl nitrite,
both of which have the same molecular formula of CH3NO2 but
different structural formulae. Nitromethane, used as a high-energy fuel for
cars, is a liquid with boiling point of 101°C. Methyl nitrite is a gas with
boiling point of -12°C which when inhaled causes dilatation of blood vessels.
Example # 2
Ethyl alcohol and dimethyl ether are two different compounds having
entirely different physical and chemical properties due to different structural
formulae owing to presence of different functional groups but have same
molecular formula. Hence they are referred as isomers of each other.
Family
or Class …………… Alcohol Ether
Structural
formula ……… CH3CH2OH CH3-O-CH3
Functional group ………… -OH -O-
Reactivity……………… …… reacts
with Na Do not react with Na
Boiling
point ………………. 78℃ 17℃
Vapour
density …………… 23 23
Molar
mass …………………. 46 46
Molecular
formula ……….. C2H6O C2H6O
Empirical
formula ……….. C2H6O C2H6O
Degree
of unsaturation … 0 0
Schematic Classification of Isomerism
Isomerism is of two types namely structural or constitutional isomerism
and stereoisomerism.
Types of Isomerism
Isomerism
is of following two types
1. Structural or Constitutional isomerism
2. Stereo or configurational isomerism
Structural
or Constitutional Isomerism
Definition
When isomerism is caused by the difference
in the arrangement of atoms or group of atoms
within molecule without
any reference to space is
called Structural or Constitutional Isomerism.
Structural or Constitutional Isomers
Structural or Constitutional Isomers are compounds that
have same molecular formula but different structural formulae due to different
arrangement of atoms or groups or multiple bonds.
Structural Isomers have entirely different
physical and in most cases also
have distantly different chemical properties.
Reason of Structural Isomerism
In this type of isomerism, compounds possessing same molecular formula
differ in their properties due to the difference
in the linkages of atoms or groups inside the molecule i.e. due to the difference
in their structures. The constitutional isomers differ in the connectivity of carbon atoms i.e. differ in their structural formulae.
Six Types of Structural or Constitutional Isomerism
Structural or Constitutional Isomerism is of following six types:
Structural or Constitutional Isomerism is of following six types:
1. Chain /skeletal/Nuclear isomerism [in all families except MSB]
2. Position
Isomerism [in all families
except alkanes, Aldehydes, acids, acid halides, MSBs]
3. Functional
Group Isomerism [in alcohol-ether,
Aldehydes-ketones, acid-ester etc.]
4. Metamerism [in ethers,
ketones, esters, secondary amines]
5. Tautomerism/keto-enol
isomerism [in Aldehydes /ketones with
enols]
6. Ring
chain isomerism
Stereoisomerism
Definition
The prefix stereo- is derived from the Greek word stereos meaning solid.
When isomerism is caused by the different
spatial configuration (i.e. three-dimensional
arrangement) of atoms or groups in space is called Stereoisomerism. Compounds having the same structural formula
but different spatial arrangement of atoms or groups in space are called
stereoisomers and the phenomenon is called stereoisomerism.
stereoisomers
Two or more compounds having same molecular
formula, same structural formula but different arrangements of atoms or groups
in space are called stereoisomers. These Compounds have same molecular and
structural formulae but different spatial arrangement of atoms or groups
Reason of Stereoisomerism
Stereoisomers have same molecular formula
and also the same
structural formula but differ in arrangement
of the bonds (atoms) in space.
Stereochemistry is the term applied to the three-dimensional aspects of
molecular structure and reactivity.
Classification of Stereoisomerism
1. Conformational isomerism
2. Configurational isomerism
There are two types of stereoisomerism:
a) Conformational isomerism. Stereo isomers which have following characteristics:
(i) Stereo isomers which cannot interconvert at room temperature due to restricted rotation known as geometrical isomerism
(ii) Stereo isomers which have different behaviour towards the plane polarized light are known as optical isomers.
b) Configurational
isomerism.
The operational distinction between Conformational
and Configurational isomers is that whether they interconvert at room
temperature or not. Conformational isomers can interconvert at room temperature
so they cannot be separated from the reaction mixture whereas configurational
isomers cannot interconvert at room temperature so they can be separated from
the reaction mixture.
Stereoisomerism is of three types:
1. Geometrical/cis-trans Isomerism
2. Optical
Isomerism
3. Conformational
Isomerism
Chain or skeletal or nuclear isomerism
Definition
Isomerism resulting from varying configuration of main carbon skeleton or chain is called skeletal or nuclear isomerism. Here a chain of minimum 4 carbon atoms is necessary to show this type of isomerism.
OR
In this type of isomerism, compounds possessing
same molecular formula differ in their properties due to the difference in the
arrangement of carbon chain i.e. branched
or unbranched chain present in
them.
Chain or Skeletal Isomers
Different compounds which have same molecular
formula but they differ in the configuration of their
main carbon skeleton or chains having
different carbon chains are called chain or skeletal isomers.
Similar chemical Properties but
different physical properties of Chain Isomers
Skeletal isomers are chemically similar because
they possess the same functional group belonging to the same homologous series
but they differ in physical properties as the van der Waal’s forces between molecules of
the straight chain isomer are much stronger than those between molecules of the
other two branched isomers.
Occurrence
Skeletal isomerism is
found in all aliphatic homologous series except mono-substituted benzenes.
Starts
with C4
Skeletal isomerism
starts with C4.
Methane, ethane and propane do not exhibit chain isomerism.
No. of possible isomers
of alkane
Examples of Chain
Isomers
(i) butane
(C4H10) has two
chain isomers. Pentane (C5H12) has the
following 3 chain isomers:
(ii) Hexane (C6H14) has
the following 5 chain isomers:
(a-b), (a-c), (a-d), (a-e),(b-d), (b-e),
(c-d), c-d) are chain isomers
(b-c), (d-e) are position isomers
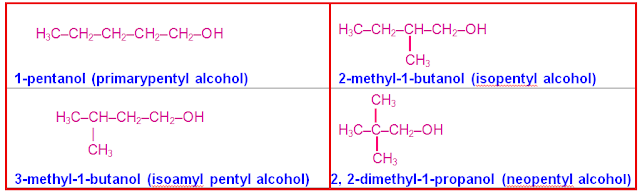
(iii) Pentyl
alcohol (C5H11OH)
has the following 4 chain isomers namely primary,
iso, isoamyl & neo.
(iv) Pentene
(C5H10) has two chain isomers namely pent-1-ene and iso-pentylene
(v) Butanal
(C4H8O) has two chain isomers
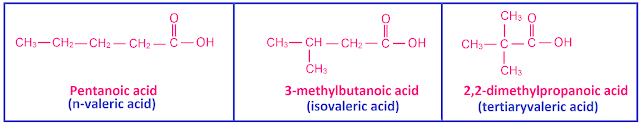
(vi) Pentanoic
acid (C5H10O2) has 3 chain isomers
Position
Isomerism
Definition
Isomerism resulting from varying
position of the functional group in
the same carbon skeleton is called positional isomerism.
OR
The compounds which have same molecular formula, same functional group, same parent carbon chain but different position
of functional group or multiple bonds or substituents show position isomerism.
Positional Isomers
Compounds which have the same structure of a carbon chain but differ
only in term of position of the multiple bonds or the functional group or
substituents are called position isomers. Position isomers belong to the same
homologous series and class of the compound
Different compounds that have same molecular formula but their
structural formulae are different due to different position of functional group
or multiple bonds in the same carbon chain are called positional isomers.
Similar chemical Properties but
different physical properties of Position Isomers
Positional isomers (belonging to the same homologous series) mostly have
same chemical properties owing
to the presence of same functional group or substituent in different positions
in the same carbon skeleton.
The physical properties of positional
isomers are different as the physical
properties like melting point, boiling point, volatility etc. are affected both
by the shapes of molecules and strength of intermolecular forces like hydrogen
bonding.
Occurrence
Positional isomerism is found in all
homologous series except benzene, mono-substituted
benzenes (MSBs), alkanes, aldehydes, carboxylic acids and their derivatives (acid
halides, acid amides, acid anhydride, sodium carboxylate, nitriles), mono-substituted
alicyclic compounds.
Minimum No. of Carbons to show Positional isomerism
Positional isomerism starts with C3 in all functionally substituted alkanes like
alkyl halides, alcohols, thioalcohols, primary amines etc.
In alkenes and alkynes,
it starts with C4.
In ketones,
it starts with C5.
Families not showing Position Isomerism
Position isomerism is never
observed in presence of chain terminating functional group like –COOH, –CHO, –COX, –CN,
etc. Aldehydes, carboxylic acid and their derivatives do not exhibit position
isomerism
Chain isomerism and position isomerism can never be
possible together
Chain isomerism and position isomerism can never be possible together
between two isomeric compounds. If two compounds are chain isomers then these
two will not be positional isomers.
Examples
(i) butene
(C4H8) has two
positional isomers namely 1-butene and
2-butene.
H3C–CH2–CH=CH2
H3C–CH=CH–CH3
1-butene/
α-butylene) 2-butene/β-butylene
(ii) Propyl alcohol (C3H7OH)
has two position isomers
namely primary and secondary;
(iii) Butyl alcohol (C4H9OH)
has three positional isomers namely primary, secondary and tertiary:
(iv) 2-pentanone and 3-pentanone are two
positional isomers of five carbon ketone.
(v) Di-substituted benzenes (DSB) have three positional isomers namely ortho, meta and para e.g. xylene (a DSB) has three positional isomers.
(vi) Cresol (methylphenol) has three
positional isomers which show different physical properties.
(vii)Dihydric phenols have three positional
isomers which show different physical properties.
(viii) There are three positional isomers of
benzdioic acids (a DSB) which have different physical and chemical properties. e.g. only
benz-1,2-dioic acid forms an acid anhydride, this being “sterically” impossible for the other two isomers
as the – COOH groups are too distant from one another.
Functional
Group Isomerism
Definition
Isomerism resulting from the presence of different functional group is
called functional isomerism.
Functional isomers
Different compounds which have same molecular formula but their
structural formulae are different due to the presence of different functional groups are called
functional group isomers.
Functional isomers belong to different homologous series and different
class
different chemical Properties and different physical properties
of Functional Isomers
Functional isomers (belonging to the different homologous series) have different chemical properties owing to the presence of different functional groups. Functional isomers also have entirely different physical properties.
Minimum No. of Carbons to show Positional isomerism
It starts with C2 in alcohol-ethers, acid-esters and
aldehydes-oxiranes,
it starts with C3
in aldehydes-ketones, ketones-oxirane.
Families not showing Position Isomerism
Alkyl halides do not show functional isomerism.
Functional and chain isomerism and functional
and positional isomerism can never be possible together
Occurrence
Important functional group isomers are given below:
Functional
Group Isomerism in Alcohol and Ethers (CnH2n+2O)
1. C2H6O
is the molecular formula of two functional isomers namely ethyl alcohol and
dimethyl ether.
2. C3H8O
is the molecular formula of two functional isomers namely primarypropyl alcohol
(or secondarypropyl alcohol) and
ethyl methyl ether.
No. of possible isomers
from given molecular formula
Functional
Group Isomerism in Aldehydes and Ketones, alkenol, oxirane and oxolane (CnH2nO)
1. C3H6O
is the molecular formula of various functional isomers namely propionaldehyde
(having aldehydic group), acetone (having keto group), propenol (unsaturated
alcohol, allyl alcohol, methyl oxirane (oxirane) and oxopropane (oxolane)
obeying general formula CnH2nO.
Functional Group Isomerism in Carboxylic acids and Esters and hydroxycarbonyl compounds etc. (CnH2nO2)
1. C2H4O2 is
the molecular formula of two functional isomers namely acetic acid (having
carboxylic group) and methyl formate (having ester group).
2. Propanoic
acid and methyl acetate (or ethyl formate) are functional isomers having same
molecular formula (C3H6O2).
Functional
Group Isomerism in Aldehydes-Ketones and Oxiranes
C3H6O is the molecular
formula of three functional isomers namely propanoic acid, propanone and
propylene epoxide.
Aromatic alcohols and phenolic compounds are
functional isomers.
Functional
Group Isomerism in Alkynes and alkadiene (CnH2n-2)
But-1-yne and 1,3-butadiene are functional isomers.
Functional
Group Isomerism in cyanides and isocyanides
Functional
Group Isomerism in Nitro and nitrite
Metamerism
Definition
Isomerism resulting from unequal distribution of carbon atoms
(alkyl or aryl groups) on either side of the polyvalent functional group is
called metamerism.
OR
It is the type of isomerism in which compounds possessing same molecular
formula differ in their properties due to the difference in the alkyl groups
present in them i.e. same functional group but different alkyl groups attached
to it. It is a type of chain isomerism.
metamers
Different compounds which have same molecular formula having same
functional group in which polyvalent atom of the same functional group joins different
combinations of alkyl or aryl radicals are called metamers.
OR
Compounds having same molecular formula, same polyvalent functional
group, same class but different alkyl groups attached to the polyvalent
functional group are metamers.
Functional groups Showing Metamerism
Polyvalent functional groups (having more than one valency) are:
Same chemical Properties and
different physical properties of Metamers
Metamers have same chemical properties due to the presence of
same functional group and belong to the same homologous series but have different
physical properties. Metamerism occurs among the members of the same
homologous series i.e. metameric pairs.
Families showing Metamerism
Metamerism is found in ethers, thioethers, ketones and secondary amines,
tertiary amines, alkenes esters etc.
Minimum No. of Carbons to show Positional isomerism
In ethers and secondary amines metamerism starts with C4.
In esters metamerism starts with C3 but in ketones
it starts with C5