Functional Groups
Definition
The functional groups are centers of chemical
reactivity. Compounds having a similar
functional group have undergone similar reactions. The presence of functional
groups enables the systematization of organic compounds into different classes.
The functional groups determine the way the molecule works both chemically and
biologically.
An
atom or group of atoms whose presence confers on organic molecule
characteristics properties unique to that group is called Functional Group. It
is the site of chemical reactivity of organic compounds.
OR
An
atom or group of atoms which is present within the organic molecule and is
responsible for its chemical behaviour and characteristic properties is called
Functional Group.
A
functional group is an atom or group of atoms, double or triple bonds whose
presence imparts specific properties to organic compounds. They are chemically
functional part of organic molecule. The hydrocarbon part i.e. alkyl group
of the molecule is usually inert, the reactivity is due to functional group.
Hence it is called active part. The functional group determines the
basic chemistry of an organic compound. It gives specific and
characteristic properties to an organic molecule, while the remainder
hydrocarbon part (alkyl group) of the molecule has an effect on its physical
properties.
For Example
(i) In alcohols (R–OH), the hydroxyl group (–OH) is functional group.
(ii) In alkyl halide (R–X), the halide group (–X) is functional
group.
Characteristics
of functional groups
1. Each functional group defines an organic
family.
2. Each functional group undergoes
characteristic reaction.
3. Function group helps us to name the
organic compounds.
4. All the compounds with the same
functional group belong to the same class.
5. Molecules can contain more than one
functional group called poly-functional compounds.
6. One functional group can modify the
properties of other functional groups.
Types
of Functional groups
1. Non-terminating FG; (without
C-containing FG)
2. Terminating FG; (C-containing FG)
Common and Important Functional Groups
Only
alkanes are devoid of any functional group. All other families possess a
characteristic functional group:
1. Olefinic Double
Bond
Olefinic double bond (i.e. carbon-carbon double bond) is the functional group of alkenes or olefins or alkylenes.
e.g.
(i) Ethene or Ethylene (C2H4) ; H2C
= CH2
(ii) Propene or Propylene (C3H6) ; CH3
– CH = CH2
(iii) butenes
or Butylene (C4H8) ; (3
isomers or 4 isomers including stereoisomers)
2. Acetylenic Triple
Bond
Acetylenic triple bond (i.e. carbon-carbon triple bond) is the functional group of alkynes or acetylenes.
e.g.
(i) Ethyne or AcetyleneC2H2 ; HCºCH
(ii)Propyne/Allylene/methyl acetyleneC3H4 ;CH3–CºCH
(iii) but-1-yne or ethyl ethyne or ethyl acetylene C4H6 ;
CH3–CH2–CºCH
(iv) but-2-yne or crotonylene or dimethyl acetylene C4H6 ;
CH3–CºC– CH3 (2 isomers)
3. Amino Group
Amino
group is the functional group of amine. They are of 3 types:
(i)Primary amines ; (–NH2) ; e.g. CH3–NH2,
CH3–CH2–NH2
(ii) Secondary amines;(>NH) ; e.g. (CH3)2.NH
(dimethyl amine)
(iii) Tertiary amine ; (àN) ; e.g. (CH3)3.N
There is a little
difference in the way amines are classified! Unlike the previous cases,
the amines are classified based on the number of carbons connected to the
nitrogen. Another difference with the amines is that the nitrogen can have four
groups connected by using the lone pair and getting a positive formal charge.
These are called quaternary ammonium salts.
4. Ether Linkage
The
linkage of two carbon atoms through an oxygen atom is called an Ether linkage
(C – O – C). It is the functional group of ether family. e.g.
(i) CH3–O–CH3 ; (dimethyl ether/Methoxymethane)
(ii) C2H5–O–CH3 ; (ethyl
methyl ether/Methoxyethane)
(iii) C2H5–O–C2H5 ; (diethyl ether/Ethoxyethane)
5. Epoxide Group
In this group, two carbon atoms are directly bonded to each other through an oxygen atom. It is the functional group of alkanepoxides or alkyl oxirane or alkylene oxide.
6. Thiol or mercapto (–SH)
It
is the functional group of mercaptan or thiol or alkanethiol.
Its type formula is RSH.
(i) CH3–SH ; Methanethiol
(ii) C2H5–SH ; ethanethiol
7. Sulphonic acid
group
It
is the functional group of sulphonic acids. Its type formula is R–SO3H.
(i) CH3–SO3H ; methane
Sulphonic acid
(ii) C2H5–SO3H ; ethane
Sulphonic acid
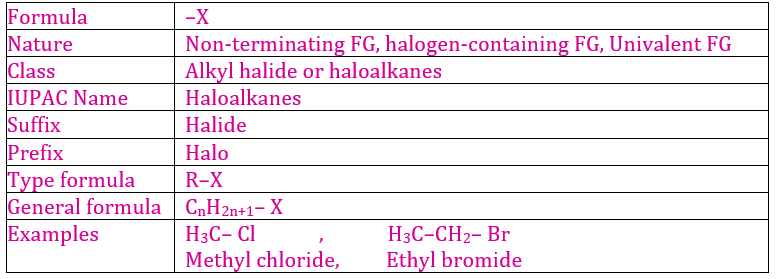
8. Halide Group
Halide
group (–X) is the functional group of two series of organic compounds i.e.
alkyl halide (R–X) and aryl halide (C6H5–X) where X
represents Cl, Br and I.
(a) Alkyl Halides
It is the series of organic compounds in which halogen atom is attached directly to aliphatic (open chain) carbon atoms. They are mono-halogen derivative of alkane.
There are 3 types of alkyl halides:
(i) primary (1°) alkyl halides ; (–CH2–X) e.g.CH3–Cl, CH3–CH2–Cl
(ii)secondary (2°) alkyl halides ; (>CH–X) e.g. (CH3)2 – CH – Cl
(iii) tertiary (3°) alkyl halides ; (àC–X) e.g. (CH3)3.C–
Cl (3°–butyl chloride)
(b) Aryl Halides
It
is the series of organic compounds in which –X atom is directly attached to
carbon atom of benzene ring. e.g.