1.1 Historical Background of Periodic Classification
Need and Search for
Classification
With the discovery of more and more new
elements, it was necessary to organize these elements systematically and need
arose for a frame work in which these elements could be classified and arranged
in in order to facilitate their study and make their study simple and
systematic. The classification of elements enabled the chemists to
understand and interpret the properties of elements in a better way.
There could be many ways
of arranging the elements; firstly they could be classified by their
states (solids, liquids or gases) at a particular temperature, secondly they
could be arranged as metals, non-metals and metalloids and thirdly one might
find patterns in their reactions with oxygen or water or other chemicals. Would one
consider trying to link these properties to the relative atomic masses of the
elements?
Previously scientist tried to
arrange the elements in a scientific, systematic and an organized
manner on the basis of their atomic weight (atomic masses) as it was
thought that the properties of elements depended upon their atomic masses (the
thought was grounded on Dalton’s atomic theory). But recently, the basis of
classification has been changed and elements are arranged on
the basis of their atomic numbers instead of their atomic masses.
Different attempts of Classification
Following attempts were made to
classify the known elements:
1. Al-Razi
Classification
2. Origin of Classification;
Dalton’s Atomic Theory
3. Dumas Work
4. Prout’s Attempt
5. Dobereiner’s Triads
6. Newland’s Law of
Octave
7. Lother Meyer’s
Classification
8. Mendeleev’s
Classification
9. Modern Periodic
table
1. Origin of Classification
The basis of classification of
elements was grounded on the Daltons’ atomic theory put forward by an English scientist, John Dalton
in 1808, according to which:
“Atoms of
different elements have different atomic masses.”
Thus it was concluded that there is
a regular relationship between atomic masses and properties of elements. “This
relationship proves to be the corner stone for the future classification of
elements”.
2. Dumas Work
Dumas (1800-1884), a French chemist arranged the elements on their combining power with chlorine.
For example, elements that combined
with 1 chlorine atom could be arranged in vertical columns in increasing order
of their atomic weights and so on.
Reasons for Failure
Dumas attempt of classification did
not gain success as all elements do not combine with chlorine and few show
variable valency.
3. Prout’s Attempt
Prout, an English chemist considered
the atomic weight of hydrogen as the basis of his classification. He considered
that:
“Atomic
weights of all elements are simple multiple of the atomic weight of hydrogen”
Reason for Failure
It could not explain the fractional
atomic weights of elements.
3.Dobereiner’s Triads
A German chemist, Johann Wolfgang
Dobereiner in 1817 noticed an interesting pattern in certain sets of three
similar elements and classified the similar elements in the groups of three
elements (in the sequence of increasing atomic mass) known as triad. He
found that the atomic mass of the middle element lay (fall) roughly half way
(midway) between the other two (i.e. the lightest and the heaviest) elements of
a triad and the elements of a triad also resemble in properties. He also noticed
that the middle elements had properties that were an average of the other two
members of a triad when arranged by the atomic weights.
e.g.
He found that the density of the
middle element in most triad is roughly equal to the average of the densities
of the other two elements. The density of strontium (2.6 g/cm3)
for example is close to the average of the densities of calcium (1.55 g/cm3)
and barium (3.51g/cm3).
He put forward Law or rule of
Triads, according to which;
“Central
atom of each set of triad has an atomic mass equal to the arithmetic mean of
the atomic masses of the other two elements.”
OR
Each set
of triad (group of three elements ordered by increasing atomic weights) has
similar properties and atomic weight of the middle element of a triad was
approximately equal to arithmetic mean (average) of the atomic weights of other
two elements of a triad”.
He arranged the elements in triads.
The elements of triad resemble in properties.
He first found alkaline earth metal
triad of Ca, Sr and Ba.
He further noticed the same pattern
for the alkali metal triad (Li, Na, K), the halogen triad (Cl, BR, I), Chalcogen
(S, Se, Te), metalloid triad (P, As, Sb) and transitional metal triad (Mn, Cr,
Fe).
Reason for failure
Dobereiner’s law of triad has a very
limited application and could not be extended to the
classification of all the elements as this rule was valid for
only very few elements. It failed as this rule was not applicable for
all elements i.e. all elements could not arrange in triads.
4. Newland’s Law of Octave
In 1864, an English (London)
industrial chemist John Alexander Newland arranged the 56 (60 or
62) known elements by order of increasing atomic weights into a table along
horizontal rows seven element long with seven vertical columns and proposed has
law of octave accordingly:
“If
elements are arranged in the ascending order of their atomic weights, the
eighth (8th) element following any given element in the series has
nearly same physical and chemical properties as first one” which means that
starting from any element, the properties of every eighth element were similar
to those of first
i.e. its
properties are a kind of repetition of the first (like the eight notes of an
octave of music or by the analogy with the seven intervals of the musical
scale).
It was compared to octaves (Sa, Re, Ga, Ma, Pa, Da, Ni, Sa) in musical
scale and thus the name Newland’s law of octaves (notes of music)
Merits
1. It arranges all 56 elements into tabular form.
2. It arranges all elements with identical properties into same group.
3. Newland’s classification of elements for the first time showed the existence of periodicity i.e. recurrence of chemical and physical properties of elements at regular intervals.
4. It also provided a great idea towards the development of
modern periodic table.
Objections
1.The Law of Octave holds up well for the first 16 (17) elements, but it failed rather badly beyond calcium in predicting a consistent trend.
2. The heavier elements could not be accommodated by this arrangement.
3. Moreover
hydrogen as not included in his table.
4. Lother Meyer’s Classification
In 1869, a German Physicist Julius
Lother Meyer (a contemporary of Mendeleev) classified the known 56
elements on the basis of their increasing atomic weights in graphical form
in nine vertical columns or groups from I to IX. Meyer’s work was based on physical
properties of elements like atomic volume. He put forward his periodic law,
which states that
‘‘physical properties of elements are
periodic function of their atomic weights’’.
The volume occupied by 1 gram
atomic weight or 1 gram atom or 1 gram mole (i.e. 6.02 x 1023 atoms)
of any element in solid state is called atomic volume which is a rough measure
of the relative sizes of atoms.
Lother Meyer’s Atomic Volume Curve
Meyer arranged the elements by plotting
a graph between atomic volumes of elements (on y-axis) against their
increasing atomic masses (on x-axis).
The plot gave a curve called Atomic
Volume Curve, consisted of sharp peak (crests) and broad
minima (troughs). The curve exhibits periodicity as similar elements
occupy same positions on the curve. For example, the highly reactive alkali
metals (Li, Na, K, Rb, Cs) occupy the peak of the curve thereby showing that
these elements have largest atomic volumes.
According to Meyer, the occupying
of similar elements on same positions on the curve was called periodicity. The
regular spacing of the highest points and occupying of similar elements
on the same positions on the curve confirmed the idea of periodicity, suggested
by Newland. [Meyer was the first scientist who considered valency as a period
property.]
Meyer’s curve showed the following
characteristics and periodicity:
1. Chemically
similar elements occupy similar position on the curves. For example; Alkali
Metals like Li, Na, K etc. occupy the peaks of the curve indicating that they
have largest atomic volumes than those of neighbouring elements while ascending
portion of the curve just before the peak is occupied by halogens showing their
smallest atomic volumes. The crest of each wave is occupied by an alkali metal
and trough by an element of small chemical affinity.
2. Alkali metals occupy
the peaks or crests of the curves.
3. Weak metals or
elements of small chemical affinity or transition metals occupy the troughs
or minima of the curve.
4. Electronegative
and gaseous volatile elements or acidic oxides forming elements are located
on the ascending portions of the curve.
5. Electropositive
or transition elements or elements with high melting points are found on
the descending portions of the curve.
6. Midway
of ascending portions of curve is occupied by halogens.
7. Midway
of descending portions of curve is occupied by alkaline earth metals.
Summary of Meyer’s Atomic Curve
Meyer’s curve shows the following
characteristics and periodicity:
Objections
Lother Meyer’s Periodic Classification
could not receive proper attention due to following reasons:
1. Meyer’s Periodic
Table was incomplete as he left no blank spaces for undiscovered
elements as compared with Mendeleev’s Periodic Table (which was characterized
by remarkable predictions of discoveries of certain elements).
2. no logical basis for classification
based on various physical properties such as atomic volume.
3. Chemical
properties of elements were completely ignored.
4. His table was non-reproducible
form of periodic table.
Mendeleev’s
Classification
Most of the credit of the
development of periodic classification of elements must go to a Russian chemist
Dmitri Ivanovitch (D.I.) Mendeleev who presented the most useful and
most systematic scheme for periodic classification of elements in March 1869. (Mendeleev’s
was notorious for cutting his hair only once a year). Up till 1869, only 63 elements
were known. Mendeleev arranged the elements in the sequence of their
increasing atomic weights. He arranged the elements of similar properties in
vertical columns and dissimilar elements in horizontal rows.
Basis of Classification
1.Increasing
order of atomic mass of elements
2.Similarity
in chemical properties of elements
Mendeleev’s work was an extension
of Newland’s octaves. The basis of his classification was the chemical
properties of elements. Mendeleev arranged the known 63 elements in the
sequence of their increasing atomic weights, placing the elements with similar
chemical properties vertically beneath each other. In his table, similar
properties occurred periodically i.e. repeated themselves at intervals as a
function of atomic weights.
Mendeleev’s Periodic Law
Since similar properties occurred
periodically as a function of atomic mass, Mendeleev stated the Periodic Law
as;
“The
physical and chemical properties of elements are a periodic function of their
atomic weights i.e. if the elements are arranged in ascending order of their
atomic weights, their properties repeat in a periodic manner.”
Features of Mendeleev’s Periodic
Table
Following are the main features of
table:
1. The elements are arranged in ascending order of their atomic masses.
2. The Mendeleev’s periodic table consisted of 8 vertical columns called groups (i.e. group I to VIII) containing similar elements and 12 horizontal rows called Series or Periods having dissimilar elements.
3. The groups are further divided into sub groups A and B. This sub division allowed him to place elements with slightly different properties in same group thereby maintaining periodicity.
4. The elements in each group have similar chemical properties but their physical properties change gradually down the group.
5. The group number indicates the highest valency of element that it can attain.
6. Mendeleev’s table clearly and forcefully proved the concept of periodicity.
7.Mendeleev’s table contained vacant spaces for
undiscovered (unknown or missing) elements with predicted atomic masses 44,
68, 72 and 100.
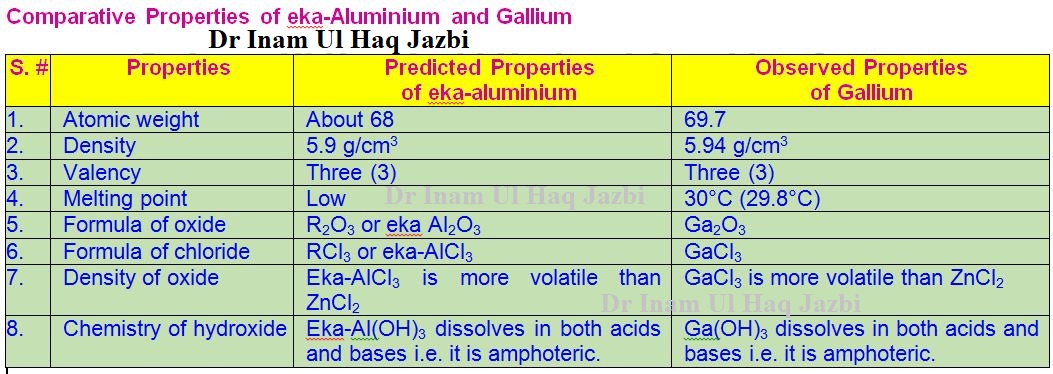
He named them eka-boron, eka-aluminium and eka silicon.
Advantages
(Merits) of Mendeleev’s Table
1. Systematic Study of
Elements
2. Special Emphasis on
Chemical Similarities
3. Determination
& Correction of Doubtful Atomic Weights
4. Separate
Group for A set of 3 Elements
5. Vacant Spaces for
Undiscovered Elements & Prediction of Properties of 3 Unknown Elements
1. Systematic Study of
Elements
Mendeleev’s table helped chemist to
study the elements more easily and systematically as it had reduced or
restricted the study of elements into a study of eight groups only. (The
study of chemistry of only one element of any group, is largely enough to predict
the properties of the other elements of the same group). For instance, the
study of sodium metal helped chemist to a large extent to predict the
properties of its other group elements like K, Rb, Cs.
2. Special Emphasis on
Chemical Similarities/ Properties take precedence over atomic weights
Mendeleev disregarded atomic masses as the only criteria for assigning places to elements because at that time, the atomic weights of many elements were not accurately known; nor was it certain that all the elements has been discovered. Great emphasis was laid on chemical similarities of elements. Thus if the properties of an element suggested that it was out of place in the sequence of atomic masses, it was placed according to its properties rather than its mass.
He placed 3 misfit pairs of
element in his table denying his own periodic law to maintain
periodicity which was supposed to be more important than following law. Hence
some elements of higher atomic mass were placed before elements of lower atomic
mass. e.g.
3. Determination & Correction of
Doubtful Atomic Weights
Mendeleev’s classification helped in correcting the doubtful atomic weights of a number of elements which has been assigned incorrect values and put them in proper places in the periodic table.
Mendeleev used the formula; Atomic weight = Equivalent weight x valency for calculating atomic weights of elements. The equivalent weight of elements can be calculated by any of the known methods and the valency can be obtained by consulting the periodic table.
For example:
(i) atomic weight of Be was correct
from 13.5 to 9. With this atomic weight, Be was given a position between
Li–7 and B–11. The properties of
Be justify this position in periodic table.
(ii) Similarly atomic
weight of indium was readjusted from
75.80 to 113. (Indium was supposed
to have the valency 2 and equivalent weight 38, so its atomic weight would be 2
x 38 = 76 and with this atomic weight it would be placed between Zn-65 and
Sr-87. There was no place between Zn and Sr. Mendeleev suggested if indium were
taken as trivalent, its atomic weight would be 3 x 38 = 114 and thus would get
the place between Cd – 112 and Sn -118 that justified its position).
(iii) Also, atomic weight of Cr that had been an atomic
weight of 43 was recalculated and found to be 52 and allocated proper place to it.
4. Separate Group for a set of 3
Elements in Group VIII
Mendeleev observed that a set of 3
elements i.e. Fe, Co, Ni, and Ru, Rh, Pd and Os, Ir, Pt
had very similar properties and could not be assigned to any particular group.
He, therefore, placed these elements at one place in Group VIII.
5. Vacant Spaces for Undiscovered Elements & Prediction of
Properties of 3 Unknown Elements
In order to maintain families of
chemically similar elements, he left blank spaces in his table for
undiscovered elements after boron, aluminium and silicon which allowed
his theory to be tested. Comparing the properties of their group elements, he
successfully predicted the three unknown elements, which he named Eka-Boron,
Eka-Aluminium and Eka-Silicon (eka means first i.e. eka-silicon means
literally first comes silicon and then comes unknown element). This prediction
helped in their discovery. By 1886, chemists had discovered all the three
elements and had been named as scandium (Sc), gallium (
Comparative Properties of eka-Aluminium and Gallium
Comparative Properties of eka-Silicon and Germanium
Defects or Demerits or Limitations of Mendeleev’s Periodic Table
1. Failure to explain
atomic structure
2. No place for isotopes of elements
3. Anomalous position of
hydrogen
4. Failure to place rare
earth (Lanthanides and Actinides) in the main body of periodic table
5. Group number does not
represent valency
6. Neglection of variable
valency
7. Unable to give cause of Periodicity
8. Anomalous or Misfit Pairs
of elements
(i) Elements Cu, Ag, Au were placed with dissimilar elements Li, Na, K, Rb, Cs.
(ii) Similar elements Cu and Hg were placed separately.
(iii) Elements of higher atomic weight placed earlier than elements of lighter atomic weights.
1. Failure to explain
atomic structure
Mendeleev’s
periodic table failed to account for atomic structure as it was based on atomic
weight and not on atomic number. Also Mendeleev’s table was silent about
electronic configuration of elements.
2. No place for isotopes of elements
No separate position has been given
to isotopes of an element having different atomic masses although the basis of
classification is atomic mass. There was no room for isotopes in Mendeleev’s
table as it was not possible to accommodate the large number of isotopes in the
periodic table.
3. Anomalous position of hydrogen
He could not
assign a correct position to hydrogen in his table. The placement of hydrogen
in group I along with alkali metals was a matter of dispute. The position of
hydrogen was not justified.
4. Failure to place rare earth
in the main body of periodic table
Lanthanides
(elements with atomic numbers 58 to 71) and actinides (elements with atomic
numbers 90 to 103) had not been placed in the main body of the periodic table.
Rather they had been given a separate position at the bottom of the periodic
table.
5. Group number does not represent valency
Group number
did not represent the valency of the elements e.g. excepting osmium, elements
in group VIII did not show a valency of 8. Also the elements in the middle of
the long periods (e.g. Mn, Cr etc.) exhibited variable valency.
6. Neglection of variable valency
Elements with variable valencies were considered to have fixed
valency.
7. Unable to give cause of
Periodicity
Cause of periodicity was not given by Mendeleev.
7. Anomalos or Misfit Pairs
of elements/ Wrong order of Some
Elements
(i). Dissimilar elements
placed in the same group
Many
elements with dissimilar properties had been placed in the same group e.g. Alkali
metals and coinage metals were place in same group in spite of their entirely
different properties. Also Mn had been placed with halogens. However
division of groups into sub-groups solved the issue later.
(ii).Similar elements
placed in different groups
Similar pairs of elements were
placed in different groups. For instance Ba and Pb resemble in many
properties but they were kept in different groups. Moreover, similar elements Cu
and Hg were also placed separately.
(iii). Misfit position of elements
of group VIII
Group VIII
has 9 elements placed in three available columns. These elements did not fit in
the system.
(iv)Position of 4 anomalous pairs of elements
Increasing
order of atomic mass could not be maintained. For placing elements in the
proper groups, certain elements of higher atomic masses precede those of
lower atomic masses in Mendeleev’s table. This was against Mendeleev’s
Periodic Law. These misfit pairs of elements were Ar-K, Co-Ni, Te-I and Th-Pa
40Ar – 39K 60Co–59Ni 127Te–126I
1.2 Modern
Periodic Table or Bohr’s Long
Form of Periodic Table
Discovery of Atomic Number
Mendeleev’s Periodic Table based on
atomic masses left many anomalies in the position of different elements in his
table. Moreover the existence of isotopes showed that the atomic mass of an
element is not the fundamental property of an element.
A British physicist, Henry Moseley
in 1914 showed that frequency of X-rays emitted by different metal anodes
varies directly with its number of protons (or electrons) or positive charge
which is called its atomic number. [He showed by investigation of X-ray spectra
of elements that this positive charge was in a definite amount and increased
regularly for one element to the next by one unit. Thus if the charge of
hydrogen nucleus is +1,then the relative charge on the nucleus of next element
helium would be +2 and that on the nucleus of third element lithium would be
+3, and so on.]
The X-ray spectra of elements showed
that the physical and chemical properties of elements depend upon the number
of electrons i.e. atomic number and their arrangement in different orbitals of
the atom. Moseley pointed out that atomic number of an element is the fundamental
property and properties of element are related to its atomic number and not
their atomic weights. Thus Moseley predicted that most of the defects of
Mendeleev’s table could be removed successfully if elements were arranged
according to their atomic numbers rather than atomic weights. Thus Moseley
modified the periodic law as:
Moseley’s Modern Periodic Law
“The
physical and chemical properties of all elements are a periodic function of
their atomic numbers
i.e. if the
elements are arranged in order of their increasing atomic numbers, the
properties of elements or similar elements are repeated after definite regular
intervals.”
With
replacement of basis of classification from atomic weight to atomic number,
many inconsistencies and irregularities in the Mendeleev’s table disappeared.
Basis of Classification
The method of arranging similar
elements in one group and separating them from dissimilar elements placing them
in periods or horizontal rows based on periodicity of elements is called
Periodic Classification of elements. It is so named as it is grounded on periodic
recurrence of physical and chemical properties of elements i.e.
periodicity.
The periodic classification of
elements is based on periodicity, due to which the elements having similar
properties are repeated at regular intervals. When elements are arranged in
ascending order of their atomic numbers, their properties show a repeating
pattern after intervals of 2,8,8,18,18 and 32. This is called
periodicity in properties. The repetition or recurrence of similar
properties among elements after specific intervals or periodically due to
repetition of similar valence shell electronic configuration is called
periodicity.
Chemical properties of elements
depend upon the number of valence electrons, hence the elements with similar
valence shell electronic configuration tend to show similar chemical behaviour.
As atomic number is related to the number of protons and number of electrons in
an atom, so the real basis of periodicity of properties is due to recurrence
of identical valence shell electronic configuration of the next element in
the same group after regular intervals of 2,8,8,18,18 and 32 in atomic
numbers].
Moseley’s Modern Periodic Law in
terms of electronic configuration
Now, Moseley’s Periodic Law may be
restated as:
“The
physical and chemical properties of elements are periodic function of the
electronic configuration of their atoms which vary with increasing atomic
number in a periodic manner”.
Long Form of Periodic Table or Bohr’s Long Form of Periodic
Table
The periodic table is an orderly
arrangement of the known chemical elements in a tabular form in
which elements are placed in the increasing order of their atomic number or
electronic structure (configuration) so that many chemical properties vary
regularly across the table.
The modern periodic table is the
result of discovery of atomic number by Moseley in 1914. The modern
periodic table based on Mosley’s Modern Periodic Law showing periodicity grounded
on Bohr’s scheme of classification of elements into 4 types depending on
the number of incomplete shells of electrons in the atom, was proposed by Rang
(1893) then modified by Werner (1905) and extended by Bury (1921) and adopted
by IUPAC in 1984 is called Bohr’s form or Long Form or Extended form of
Periodic Table consisting of groups and periods (because it contains 16 groups
or 18 vertical columns rather than 8 and 7 periods instead of 12) .
With replacement of basis of
classification from atomic weight to atomic number, many inconsistencies and
irregularities in Mendeleev’s table disappeared.
Difference
between Mendeleev’s periodic table and Modern periodic table
1. Modern periodic
table is based on the most fundamental property, atomic number of elements, while
Mendeleev’s periodic table is based upon
the atomic masses of elements.
2. Modern periodic
table explains clearly why elements in a group display similar properties and
elements of a group differ in properties from elements of other groups. Mendeleev’s
periodic table failed to do so.
3. In Mendeleev’s
periodic table, there are several anomalies e.g. the position of isotopes,
wrong order of atomic masses of some elements etc. In the modern periodic
table, these anomalies have been removed.
4. In
the long form of the peridoc table, elemetns have been cleraly separated
as representative elements, transtion elements and noble gases. Metals and
nonmetals are aslo separated. But in Mendeleev’s periodic table there is
no such separation of different types of elements.
5. In the modern
periodic table the subgroups A and B are clearly separate because the
elements belonging to subgroup A differ in properties from those of elements
belonging to subgroup B. In Mendeleev’s periodic table, the two subgroups are
kept together.
Applications of Modern Periodic Table
1. Prediction of Properties of element in a group.
2.Prediction of Molecular formula of compounds (between
elements of different groups).
3.Prediction of new or unknown elements has been
possible.
4. Visualization of Reactivities
of elements.
5.Suggestions for further
research become available.
Merits or Advantages of Long Form of Periodic Table
1. Controversial
Position of Hydrogen
2. Disputed Position of Helium
3. Controversial
Position of Rare Earths
4. Three Columns in group
VIIIB
5. Gaps
in the Periodic Table
6. Some Properties Neglected
Merits of Long Form of Periodic Table
1. Placement of elements according
to fundamental property, atomic number
The modern
periodic table is based on more fundamental property, atomic number. It relates
to fundamental property i.e. atomic number.
2. Relation of Properties of Element with
Electronic Configuration
It relates the position of an element to
its electronic configuration.
It explains
why all the elements in a group have identical chemical properties while the
elements in a period have different chemical properties.
All the
elements in a group have similar properties because they have similar valence
shell electronic configuration. On the other hand, all the elements in a period
have different properties because thyme have different valance shell electronic
configuration due to progressive addition of electrons to the valence shell on
moving across a period.
3. Defects of Mendeleev’s Table Removed
The anomalous pair of
elements i.e. Ar-K, Co-Ni, Te-I and Th-Pa are found arranged rightly in the
table when they are placed in the order of increasing atomic numbers.
4. Position of Isotopes Solved
Different isotopes of an element have been occupied one and the same place in the periodic table as they have same atomic numbers.
5. Exhibition of Distinct
Periodicity and Cause of periodicity
It clearly exhibits
periodicity in properties of elements i.e. recurrence of elements with similar
properties. According to modern periodic table cause of periodicity is
recurrence of elements with similar outer shell configuration.
6. Demonstration of various types of elements
It clearly illustrates active metals, non-active metals, transition metals, metalloids, non-metals & noble gases.
7. Division of Elements into
Four Blocks
It divides the elements into 4 blocks i.e.
s, p, d and f-blocks.
8. Separate Position of A and B Groups
The elements of the two
sub-groups have been placed separately and thus dissimilar elements do not fall
together.
9. Showing Trend in Chemical
Properties
It clearly
brings out the trend in chemical properties in a period and group.
10. Simple, systematic
and Easy Study of Elements
It makes the
study of the properties of elements (and their compounds) simple and easy. It systematizes the study of elements. In the periodic
table, elements with similar properties have been placed in the same vertical
columns or groups. If we know the properties of one element of the group, the
properties of other elements in the same group can be predicted. Thus, there is
no need of studying the properties of all the elements.
11. Memorable and Reproducible Form of Periodic
Table
It is easy to remember, understand and
reproduce.
12. Prediction of Properties of element in a group
It is
possible to predict the properties of an element from the location of the
element in the periodic table. For example, if the element belongs to the group
IA, it is likely to be a reactive metal. If the element is the last element of
the period, it would be a gas which is almost inert.
Demerits
of Modern Periodic Table
1. Controversial Position of Hydrogen
2. Disputed Position of
Helium
3. Controversial Position of Rare Earths
4. Three Columns in group VIIIB
5. Gaps in the Periodic Table
6. Some Properties Neglected
1. Controversial Position of Hydrogen
Position of hydrogen in
Group IA is disputed and thus its exact position is yet not decided and still
remains unsolved.
2. Disputed Position of
Helium
Position of helium in
VIIIA group is controversial (configuration of He is 1s2 whereas the
configuration of other noble gases is ns2np6).
3. Controversial Position of Rare Earths
Lanthanides and
actinides have still not been adjusted in the main body of the periodic table.
4. Three Columns in group VIIIB
Group VIIIB consists of three columns.
5. Gaps in the Periodic Table
There are large gaps in
the periodic table e.g. group IIA is widely separated from group IIIA.
6. Some Properties Neglected
Some properties of
elements such as specific heats have no relationships with the periodic classification.
1.3 Groups,
their Sub-Division and their General Features
Definition and Sub-Division
The vertical columns of elements in the periodic table are
called Groups. A group consists of a set or series of elements having identical
valance shell configuration. Periodicity of properties of
elements gives rise to groups of the periodic table.
All the elements belonging to the same group constitute a family.
All the elements belonging to a particular group have same number of a valence
electrons and hence exhibit similar properties.
Total number of groups
There are 18 vertical columns in the modern periodic table,
so there are 18 groups in the modern periodic table which are numbered
from 1 to 18 according to recommendations of IUPAC.
Earlier, the designation of these groups was the same as in the
Mendeleev’s periodic table. Thus formerly, there were eight groups (I to
VIII) but each group is further sub-divided into A and B sub-groups. But the total
groups including A and B sub-groups are 16 as group VIIIB consists
of three columns.
The relationship between the two ways of numbering the group is
given below:
Groups are numbered by Roman numericals
as IA, IIA, IIIB to VIIIB (comprising of three columns), IB, IIB, IIIA to VIIIA
or zero group (or by simple numericals as 1, 2, 3, ……….. 16,
17, 18).
Types of Groups
Groups are divided into A-family
and B-family. The elements of
A-family are chemically different from the elements of B-family.
The elements of sub-group A or the elements of groups 1, 2,
13, 14, 15, 16, 17, and 18 are called Main group or Normal or Major or Representative
Elements as the properties of these elements are represented by valence
electrons. These elements have all their inner shells complete. Their only
the outermost shell is incomplete. In these elements the outermost shell
gets progressively filled from group 1 to group 18 as we move from left to
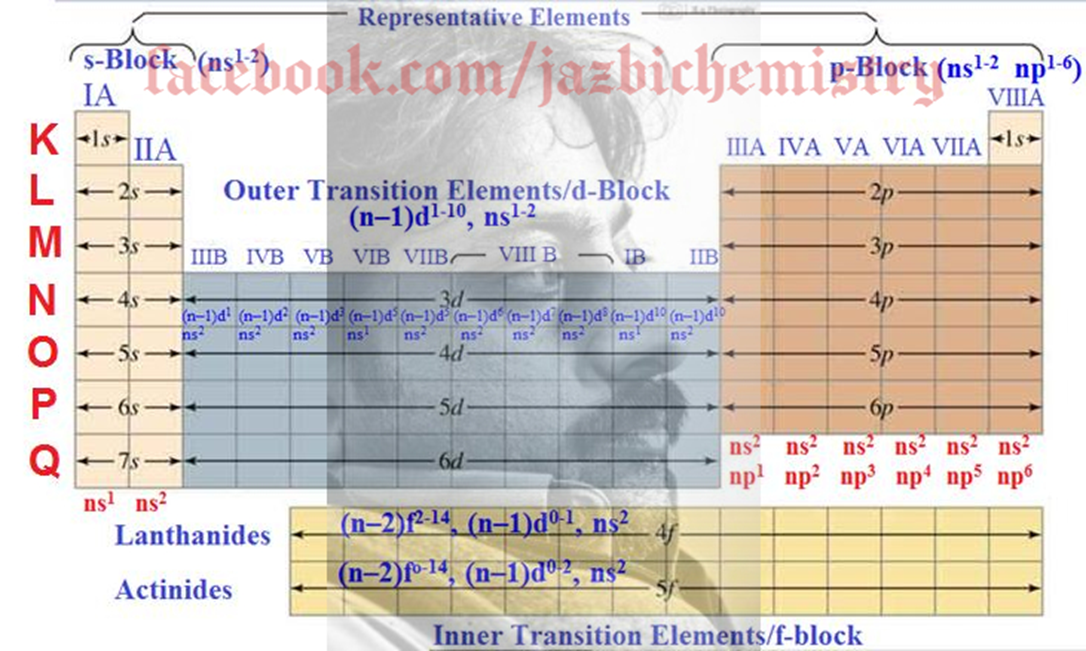
right in a period. The elements of group IA and IIA are called s-block elements
(which include active metals) while the elements of group IIIA to VIIIA are
called p-Block elements (which include all non-metals, metalloids and weak
metals). The group VIIIA is also called zero group which contains noble gases.
The elements of sub-group B or the elements
of groups 3 t 12 are called
Transition (or outer transition) elements because the properties of these
elements show a gradual change or transition between the two sets of
representative (s and p-block) elements, on either side of them. In these
elements the outermost and the penultimate (next to outermost) shells are
incomplete. All transition elements are metals.
Lanthanides and actinides are
collectively known as inner transition elements. In these elements, the
outermost three shells are incomplete.
General Characteristics of Groups
1. Representation of Total Valence Electrons
Group number of an element
represents the total number of valence electrons in its valence shell e.g.
oxygen belongs to VIA group as it has six valence electrons.
2.Representation of Maximum Valency and the highest Oxidation
State
Group number of an element
represents its maximum valency and the highest oxidation states. (Group number
of an element is equal to its valency with respect to oxygen).
3. Exhibition of Identical Valence Shell Electronic
Configuration
Elements of a same group have
identical valency shell electronic configuration.
4. Exhibition of Same Chemical Properties
Elements in same group show same
chemical properties due to same valency shell configuration.
5. Exhibition of Regular Gradation in Physical Properties
Elements of a group show regular
gradation (change) in its physical properties on descending a group due to
gradual change in their atomic sizes and electronegativities.
6. Different Behaviour of First Congeners of Each Group
The first member of each group shows
slightly different behaviours from other members of that group due to its small
atomic size.
7. Increasing Electropositivity and Decreasing
Electronegativity
Electropositivity (metallic
character) increases while electronegativity decreases down each group with
increasing atomic numbers due to increasing atomic size.
8. Identical number of
Valence electrons and valence orbital on descending a group
On moving down a group, the number of shells increase but the total
number of valence electrons and valence orbital remain same.
9. Difference in
Properties of Sub-groups A and B
The elements of A-family are
chemically different from the elements of B-family.
10. Representative and Transition elements
The elements of family A (IA to VIIA) are called normal or representative
elements having ns1 to ns2 np5 valence shell
electronic configuration. Elements of group VIIIA are called inert or noble
gases with valence shell configuration of ns2 np6.
The elements of family B (IB t VIIIB) are called outer transition
elements or d-block elements as outer electrons fall in (n-1)d-orbital.
Groups Learning Key/ Mnemonic for learning groups
Group IA or
Lithium Family (Alkali Metals)
Members
This group includes lithium (3Li),
sodium/natrium (11Na), potassium/kalium (19K), rubidium (37Rb),
cesium (55Cs) and francium (87Fr). Francium is
radioactive. Sodium and potassium are the 6th and 7th
most abundant elements in the earth crust with % abundance of 2.6% and 2.4%
respectively.
Groups Learning Key/ Mnemonic for group IA
General Characteristics
1. Electropositive Nature, High Reactivity,
Solid State and Low Volatility
2. valence
shell electronic configuration
of ns1 , monovalent
Nature, Fixed Oxidation state of
+1
3. Formation of Monovalent Cation
4. Reducing Behaviour
5. Forming only Ionic bonds
6. Basic Nature of Oxides
7. Trend of Physical Properties
1. Electropositive Nature, High
Reactivity, Solid State and Low Volatility
They are highly reactive and
strongly electropositive metallic elements relatively soft solids having low
melting and boiling point.
2. valence shell electronic configuration of ns1 , monovalent Nature, Fixed Oxidation state of +1
They are associated with valence
shell electronic configuration of ns1 (where n is the number of
orbits ranges 2-7) showing that they contain only 1 valence electron, so they
are monovalent, exhibiting a fixed oxidation state of +1.
3. Formation of Monovalent
Cation
They have tendency to lose their
single valence electron on reaction to get respective inert gas like
configuration of the previous period to form monovalent positive ion (M+)
showing their electropositive character.
4. Reducing Behaviour and
Low I.P
They
are powerful reducing agent due to their low ionization energies.
5. Forming only Ionic bonds
They
can form only ionic bonds.
6. Basic Nature of Oxides
They themselves, their oxides, hydroxides, hydrides, peroxides are basic in nature and when dissolve in water forming alkalis. That is why they are known as alkali metals.
7. Trend of Physical
Properties
They have largest atomic size in
their respective period. Their atomic radii, ionic radii, atomic volumes
increase down the group from Li to Cs due to the addition of extra shell to
each element and due to same reason melting and boiling points decrease while
electropositivity increases downward.
Group IIA or Beryllium Family
(Alkaline Earth Metals)
Members
This group comprises of Beryllium (4Be),
magnesium (12Mg), calcium (20Ca), strontium (38Sr),
barium (56Ba) and radium (88Ra). Radium is radioactive.
Calcium is the 5th most abundant element with % abundance of 3%
while magnesium is the 8th most abundant element with % abundance of
2% in the earth crust.
Groups Learning Key/ Mnemonic for group IIA
General Characters
1. Electropositive Nature, High Reactivity,
Solid State and Low Volatility (but less than alkali metals)
2. valence
shell electronic configuration
of ns2, divalent
Nature, Fixed Oxidation state of
+2
3. Formation of Monovalent Cation
4. Reducing Behaviour
5. Forming only Ionic bonds
6. Basic Nature of Oxides
7. Trend of Physical Properties
1. Electropositive Nature,
High Reactivity, Solid State and Low Volatility (but less than alkali metals)
They are reactive and
electropositive metallic elements but less reactive and less electropositive
than alkali metals, a bit harder having relatively high melting and boiling
points than the alkali metals.
2. valence shell electronic configuration of ns2, divalent Nature, Fixed Oxidation state of +2
They have valency shell electronic
configuration of ns2 showing that they contain two valence
electrons, so they are divalent exhibiting a fixed oxidation state of +2.
3. Formation of divalent
Cation
They have tendency to lose their
both valence electron on reaction to get respective inert gas like
configuration of the previous period to form divalent positive ion (M2+)
showing their electropositive character.
4. Reducing Behaviour and
high hydration energy
They are powerful and more stronger reducing
agents than alkali metals because of high hydration energy of M2+
ions.
5. Forming only Ionic bonds
except Be
They form ionic bond except Be and
Mg (however Mg can form some ionic compounds like MgO, MgSO4).
6. Basic Nature of Oxides
Their oxides are basic giving weak
alkaline solution on dissolution in water. That is why they are called alkaline
earth metals as these metals exist as their oxides (lime; CaO, strontia; SrO;
Baryta; BaO) in the earth’s crust and are alkaline in nature.
7. Trend of Physical
Properties
They have relatively smaller atomic
radii, ionic radii and atomic volumes due to their greater nuclear charge.
However down the group they do not show a systematic and regular trend in
melting points, boiling points and densities.
Group IIIA or
Group 13 (Boron Family/Triels)
Members
This group includes boron (5B),
aluminium (12Al), gallium (31Ga), indium (49In)
and thallium (81Tl). Boron is a metalloid showing dual
characteristics of both metals and non-metals while rest of them are weak
metals. Aluminium is the 3rd most abundant element in the
earth’s crust with % of abundance of 7% (7.6%).
Groups Learning Key/ Mnemonic for group IIIA
General Characteristics
1. Electropositive Nature, High Reactivity,
Solid State and Low Volatility (but less than alkali metals)
2. valence
shell electronic configuration
of ns2 np1, trivalent
Nature, Common Oxidation state of
+3
3. Formation of Monovalent Cation
4. Reducing Behaviour
5. Forming only Ionic bonds
6. Acidic Nature of Oxides
7. Trend of Physical Properties
1. Electropositive Nature,
Low Reactivity, Solid State and Low Volatility
Except boron, they are highly
electropositive elements showing metallic character which increases down the
group due to increase in atomic size (or atomic volume) relatively hard having
high melting and boiling points (except Ga with m.p= 29.9○C).
2. valence shell electronic configuration of ns2 np1, trivalent Nature, Common Oxidation state of +3
They are associated with ns2
np1 valency shell configuration i.e. they contain 3 valence
electrons, so they are trivalent showing a valency of 3 exhibiting a most
common oxidation state of +3. Later members also show 1 valency and +1
oxidation state due to inert pair effect.
3. Formation of Trivalent
Cation
Besides boron, they have tendency to
lose three valence electrons acquiring noble gas configuration to form
trivalent positive (M3+) ions showing their metallic behaviour.
|
|
|
|
|
|
|
|
|
4. Reducing Behaviour
They are reducing agent especially aluminium powder.
5. Forming only Covalent
bonds
They preferably form covalent bond. However
some ionic compounds of aluminium are known like Al2O3,
Al2(SO4)3.
6. Basic Nature of Oxides
They mostly form acidic oxides
except Al which forms amphoteric oxide.
Group IVA or Group 14 (Carbon Family/Tetrels)
Member
This group includes carbon (6C),
silicon (14Si), germanium (32Ge), tin or stannum (50Sn)
and lead or plumbum (82Pb). Of these elements carbon is a typical
non-metal, silicon and germanium are metalloids and tin and lead
are metals.
Carbon is the 16th (14th
or 17th in some books) most abundant element in the earth crust
(0.18%) and in human body carbon is the 2nd most abundant element (18%).
Silicon is the 2nd most abundant element (26%) in the earth crust.
In this group, there is smooth
transition from non-metal to metal through metalloid. This group occupies
the middle part of the periodic table and forms a link between more
electropositive and more electronegative elements.
Mnemonic For Group IVA
General Characteristics
1. Nature, Low Reactivity, Solid State and Low
Volatility
2. valence
shell electronic configuration
of ns2 np2
3. Formation of Cation
4. Reducing Behaviour
5. Forming only Ionic bonds
6. Acidic Nature of Oxides
7. Trend of Physical Properties
1. Nature, Low Reactivity,
Solid State and Low Volatility
They all are monoatomic solids elements having high
melting and boiling points.
2. valence shell electronic configuration of ns2 np2
They are associated with valency
shell configuration of ns2 np2
showing that they contain 4 valence
electrons and so they are mostly tetravalent showing a valency of 4.
Sn and Pb exhibit a variable valency of 2 and 4 due to inert pair effect. Carbon
exhibit a variety of oxidation states in organic compounds like –4, –2, –1, +2,
+4, 0 etc.
3. Electronegative and electropositive character
and Formation of Cation
Only carbon can form anions like
carbide ion (C4–) or dicarbide ion (C2– or [Cº]2–). Ge, Sn and Pb form
divalent and tetravalent cations like Sn2+, Sn4+, Pb2+,
Pb4+ due to inert pair effect.
4. Forming both covalent and
Ionic bonds
First three elements C, Si and Ge
form covalent compounds while Sn and Pb preferably form ionic compounds. The
nature of the compounds M2+ and M4+ cations can be
predicted by Fajan’s rule which states that smaller the cations, the greater
would be the covalent character. In general, compounds M4+ are
covalent while that of M2+ are ionic in nature.
5. Exhibition of allotropy
Except lead, all elements exhibit
the property of allotropy e.g. carbon exists in a variety of allotropic forms
like crystalline forms such as diamond, graphite and amorphous forms such as
coal, etc. Silicon exists in crystalline and amorphous forms. Tin is found as
grey tin (diamond type structure), white tin (tetragonal crystals) and brittle
tin (rhombic crystals).
6. Acidic Nature of Oxides
Oxides of C and Si (CO2
and SiO2) are acidic.
7. Trend of Physical
Properties
Down the group, atomic radii and
atomic volumes increase due to addition of a new shell and from the same reason
metallic character increases down the group. Thus Sn and Pb are typical metals.
Group VA or
Group 15 or Nitrogen Family (Pnictogens/Pnicogen)
Member
This group contains nitrogen (7N),
phosphorus (15P), arsenic (33As), antimony or stibium (51Sb)
and bismuth (83Bi). Among these elements, N and P are non-metals,
As and Sb are metalloids and Bi is a metal.
The group VA elements are
also called pnictogens
(pnico=suffocation ; gens = producing) due to specific smell of nitrogen and
other. The term pnictogen (or pnicogen) is derived from
the Ancient Greek word pnigein
meaning "to choke or stiffle", referring to the choking or
stifling property of nitrogen gas in absence of oxygen. It can also be
used as a mnemonic for the two most
common members, P and N. The term "pnictogen" was suggested by the
Dutch chemist Anton Eduard van Arkel in the early 1950s. It is also spelled "pnicogen".
Nitrogen is the 10th most
abundant element (0.6%) in the earth crust constituting fourth-fifths (4/5) of
the air (78%).
Phosphorus is the 12th
most abundant element in the earth crust (0.2%).
Both N and P are essential to living
organisms.
This group is often selected for
systematic studies because among its members there is essentially a regular
change with atomic weight and size from characteristics of a true non-metal (N)
to a typical metal (Bi). There is a large variation of properties in going down
the group.
Mnemonic For Group VA
General Characteristics
1. Nature, State, volatility and atomicity
Nitrogen exists as diatomic
molecules (N2), phosphorus as tetraatomic molecule (P4)
while rest of them exists in monoatomic form. Nitrogen is gas while all other
members are solid.
2. valence shell electronic configuration of ns2 np3
They have valency shell
configuration of ns2 np3
i.e. they have total 5 valence
electrons exhibiting a variable valency of 3 and 5 so they are mostly trivalent
or pentavalent except Bi which exhibit fixed valency of 3. However nitrogen
shows a variety of valencies of 1, 2, 3, 4 and 5. Phosphorus also exhibits more
than one valency 1, 3, 4, 5.
3. Electronegative and electropositive character
and Formation of Cation
Only nitrogen and phosphorus have
tendency to gain 3 electrons to form nitride ion (N3–) and phosphide
ion (P3–) respectively due to their small atomic size and large
ionization potential.
4. Forming both covalent and
Ionic bonds
They preferably form covalent
bond. However metallic nitrides and phosphides are mostly ionic.
5. Acidic Nature of Oxides
They form
all the three types of oxides. Nitrogen forms a variety of oxides which
are either acidic or neutral e.g. NO, N2O, NO2, N2O4,
N2O3 and N2O5.
6. Exhibition of allotropy
All of the elements except nitrogen
exhibit the property of allotropy e.g. P has two allotropes mainly white and
red phosphorus.
Group VIA or
Group 16 or Oxygen Family (Chalcogens)
Members
This group consists of oxygen (8O),
sulphur (16S), selenium (34Se), tellirium (52Te)
and polonium (84Po). Of these elements, O and S are non-metals,
Se and Te are metalloids and polonium is metal.
This group is also known as
chalcogens meaning the ore-forming elements because most the ores of
metals occur in nature as oxides and sulphides.
oxygen is the
most abundant element in the earth crust (50%) and it is also the most abundant
element in the human body (65%) while constituting one-fifth (1/5) of the air
(21%).
Sulphur is the 16th most
abundant element (0.04%).
Both oxygen and sulphur are
essential to living organisms.
There is a large variation of
properties in going down the group.
Mnemonic For Group VIA
General Characteristics
1. Nature, state, volatility and atomicity
2. valence
shell electronic configuration
of ns2 np4
3. Electronegative and electropositive character
and Formation of Cation
4. Forming both covalent and Ionic bonds
5. Acidic Nature of Oxides
6. Oxidizing property
7. Exhibition of allotropy
8. Trend of Physical Properties
1. Nature,
state, volatility and atomicity
Oxygen is a gas found as diatomic
molecule (O2), other memebrs are solids which exist as octamolecule
(S8, Se8) or monoatomic form (Te, Po). However liquid
sulphur may exist in S8, S6, S4 forms.
2. valence shell electronic configuration of ns2 np4
They have valency shell
configuration of ns2 np4
showing that they have 6 valence
electrons exhibiting a variable valencies of 2, 4 and 6 (except oxygen which cannot exceed its valency to 2). Most
common valency is however 2. Their most common oxidation state is -2.
But they also show variable oxidation states.
3. Electronegative and electropositive character
and Formation of Cation
Only oxygen and sulphur have
tendency to gain 2 electrons to form bivalent anions namely oxide (O2–)
and sulphide (S2–) ions respectively due to their small atomic size
and high electron affinity. Oxygen can also form peroxide [O22–]
and superoxide ion [O21–]
4. Forming both covalent and
Ionic bonds
They preferably form covalent
bond. However metallic oxides, peroxides, superoxides and metallic
sulphides are mostly ionic.
5. Acidic Nature of Oxides
They form acidic oxides. E.g. SO2, SO3, SeO2
etc.
6. Oxidizing property
They
are oxidizing agents.
7. Exhibition of allotropy
All the elements exhibit the
property of allotropy e.g.
(i) oxygen has two allotropic forms namely ordinary molecular
oxygen (O2) and trioxygen or ozone (O3).
(ii) Similarly sulphur has a number
of different allotropes like rhombic, monoclinic and plastic sulphur.
8. Trend of Physical
Properties
Metallic character, ionic and basic
nature increase regularly down the group.
Group VIIA or
Group 17 or Halogens (Fluorine Family)
Members
This group comprises of fluorine (9F),
chlorine (17Cl), bromine (35Br), iodine (53I)
and astatine (85At). Except astatine which is a metal and
radioactive all others are non-metals.
They are called halogens, a term
which means salt formers because they form salts with metals called
Halides.
Mnemonic
for group VIIA
General Characteristics
1. Electronegative Nature,
High Reactivity, any State and high Volatility
2. valence shell electronic configuration of ns2 np5
3. Electronegative character
and Formation of Univalent anion
4. Oxidizing Behaviour
5. Forming Ionic bonds and
covalent bonds
6. Acidic Nature of Oxides
7. Trend of Physical
Properties
1. Electronegative Nature,
High Reactivity, any State and high Volatility
They are highly reactive and
strongly electronegative non-metallic elements (active non-metals).
At room temperature fluorine and
chlorine are coloured gases, bromine is a volatile liquid and iodine is a dark
coloured sublime solid.
They exist as diatomic molecules
i.e. F2, Cl2, Br2, I2 except At.
They are found as discrete molecule held together by van der Waal’s forces which
accounts for their volatile nature.
2. valence shell electronic configuration of ns2 np5
They are associated with valency
shell configuration of ns2 np5
showing that they have 7 valence
electrons exhibiting a most common valency of 1 and are mostly univalent.
However except F, all exhibit a variety of valencies of 1, 3, 5, 7. Their most
common oxidation state is -1. However they can also show positive oxidation
states of +1, +3, +5 and +7 except fluorine.
3. Electronegative character
and Formation of Univalent anion
They have tendency to accept an
electron easily attaining the next noble gas configuration to form univalent
halide ions (i.e. X1– e.g. F–, Cl–, Br–,
I–) due to their high ionization energies and large negative
electron affinities.
4. Oxidizing
Behaviour
They are powerful oxidizing agent due to their smallest atomic
radii and high electron affinities.
5. Forming Ionic bonds and
covalent bonds
They can form ionic as well as covalent bonds.
6. Acidic Nature of Oxides
Their oxides are acidic in nature.
The strength of acidic nature of oxides increases with the increase in
oxidation state of halogen but decrease on descending a group.
7. Trend
of Physical Properties
They have smallest atomic size in their respective period.
Group VIIIA or
Group 18 or Zero Group or Aerogens (Inert or Noble Gases)
Members
This group includes helium (2He),
neon (10Ne), argon (18Ar), krypton (36Kr),
xenon (54Xe) and radon (86Rn). Radon is radioactive.
The clue of existence of noble gases
was provided by Cavendish in 1785.
The first noble gas discovered by an
English scientist Ramsay (and Raleigh) in 1892 (1894) was argon
(Greek meaning idle or lazy) from air.
In the same year, Ramsay isolated the lightest of all
noble gases helium (meaning the Sun)
from uranium ores.
During 1898, Ramsay and Rayleigh
and travers isolated three
additional noble gases, Neon (new), Kr (hidden) and Xe (stranger) from air.
Noble gases are found in the
atmosphere in very small quantities. As these elements were not known at the
time of Mendeleev, so no place was kept for them in the periodic table. They
had, therefore, been placed in an additional group called zero group which was
inserted in between the most electronegative halogens and group VII and the
most electropositive alkali metals of group I.
% of Noble gases in Air
Mnemonic
for group VIIIA
General Characters
1. Nature, State, Volatility, Atomicity
2. valence shell electronic configuration of ns2 np6 and valency
3. No electropositive or
electronegative Character
4. Inertness
1. Nature, State, Volatility, Atomicity
They are monoatomic
and low boiling point, diamagnetic, colourless, odourless and tasteless gases.
They do not resemble with any other elements in the periodic table either to
the left or right. Thus they act as a bridge between electronegative and electropositive
elements in the periodic table.
2. valence shell electronic configuration of ns2 np6 and valency
They are
associated ns2 np6 (where n = 2 – 6) outer shell electronic
configuration is i.e. they have 8 valence electrons showing that they have
completely filled outer shell (or fully filled s and p sub-shells or orbitals)
except helium which has only two valence electrons (i.e. 1s2).
All noble
gases are non-valent elements (i.e. possess zero valency indicating that
they have no combining tendency with other elements) which is attributed due to
completely filled valence shell.
3.No electropositive or
electronegative Character
They have
complete valence shell in the form of either complete octet (ns2 np6)
or complete duplet for He (1s2) of electrons in their valence shell.
Due to completely filled valence shell, they are exceptionally stable and are
unable to gain or lose electron due to zero electron affinity and very high I.P
respectively. No atom has complete outer shell with the exception of He and
Ne).
All atoms
tend to get inert gas like configuration in order to acquire stability. Thus
they are used as standard for comparing electronic configuration of elements.
4. Inertness
They are
chemically inert or non-reactive due to completely filled outer s- and
p-sub-shells (orbitals). Their inertness is attributed due to their small
atomic volumes, high I.P and zero electron affinity.
The word
inert is strictly used for He, Ne and Ar but should not be used for Kr, Xe and
Rn as they have large atomic volumes and thus form few compounds like KrF2,
XeO2 etc under drastic conditions.
Periods,
their General Features and their types
Definition of
Periods
The horizontal rows of elements in
periodic table arranged in the ascending order of their atomic numbers are
called Periods which are designated by simple numericals. In each period, the
elements have been placed in the increasing order of their atomic numbers.
There are 7 periods in the
periodic table. Period second and third are called short periods while 4th
and 5th are called long periods and 6th periods is
called longest periods and 7th period is called incomplete
period. Elements of period 1 and 2 are called typical elements.
Recently period 1 is called shortest
period, 4th and 5th periods are called longs periods, 6th
period is termed as longest period and 7th period is referred as
incomplete period.
|
|
General Features
of Periods
2. Period
number of an element represents the total number shells in that element
e.g. Iron belongs to fourth period as it has 4 electronic shells in its atom.
The period indicates the value of n for the outermost or valence shell.
The period
of an element can be predicated by observing the electronic configuration of
the element. The period of the element is same as the number of the valence
shell. For example, if in an element third shell is the valence shell, then it
belong the third period (period 3).
3. Each period starts with the filling of electrons in a new
energy level (quantum shell) and continues till the p-orbital of the same
shell.
4. Each period starts with an alkali metal (except 1st period which begins with
hydrogen) with one valence electron and ends up with a noble gas (except 7th period) with 8 valence electrons
except He which has only 2 electrons.
5. In each period
(especially short periods), the valency of elements with respect to
hydrogen increases from 1 to 4 and then falls from 3 to 1.
6. All the elements in
a period have different valence shell configuration and hence have different
chemical properties. The elements within a period have dissimilar properties
from left to right across any period.
7. The physical and
chemical properties of elements change from metallic to non-metallic
along each period.
8. Electropositive
elements (metals) are at far left side while
electronegative elements (non-metals, gases, metalloids) are at the right side of table.
9. Metallic character
decreases while non-metallic character increases from left to right across each
period. e.g. Na is a metal while Cl is purely a typical non-metal.
10. Atomic volume or
atomic radius (i.e. size of atom) decreases from left to right across each
period.
11. The number of
elements in each period is twice the number of atomic orbitals available in the
energy level that is being filled. There are 2 elements in the 1st
period, 8 in the 2nd, 8 in the 3rd, 18 in the 4th,
18 in the 5th, 32 in the 6th and 7th period is
incomplete.
Short and Long Periods
Period 2 and 3 are called short
periods while period 4, 5 and 6 are called long periods. Elements of period 1
and 2 are called typical elements.
First Period (Shortest Period)
It is the shortest period of the periodic
table containing only two elements hydrogen and helium (both of them are
gaseous non-metals). This period corresponds to filling up of K-shell.
Second and Third Periods (Short
Periods)
Period 2 and 3 are called Short
Periods each contains 8 elements (2 s-block and 6 p-block elements) from 3Li to 10Ne
and 11Na to 18Ar respectively.
Period 2 signifies the filling up of
L-shell (containing two energy
levels 2s and 2p) and period 3 corresponds to filling up of M-shell up to 8 electrons (containing
two energy levels 3s and 3p).
Period 2 includes Li, Be, B, C, N, O, F & Ne while
period 3 includes Na, Mg, Al, Si, P, S,
Cl & Ar.
Their general valence shell
configuration is ns1 np0 to ns2 np6.

Fourth and Fifth Periods (Long
Periods)
Period 4 and 5 are called Long
Periods each contains 18 elements (2 s-block, 6 p-block and 10 d-block
elements) from 19K to 36Kr and 37Rb
to 54Xe respectively.
Period 4 corresponds to filling up
of N-shell and also M-shell
(containing three energy level 4s, 3d, 4p i.e. period 4 starts with filling up
of 4s orbital followed by 3d and finally 4p orbital) while period 5 signifies
the filling up of O-shell and also
N-shell (containing three energy levels 5s, 4d, and 5p i.e. period 5 starts
with filling up of 5s orbital followed by 4d and finally 5p orbital).
Out of 18 elements, 8 elements (two
s-block and eight p-block elements) are representative elements and the
remaining ten are transition elements. Fourth period contains 10 transition
elements (called 1st transition series) from Scandium to Zinc i.e. 21Sc
to 30Zn. Fifth period also contains 10 transition elements (called 2nd
transition series) from Yttrium to Cadmium i.e. 39Y to 48Cd.
Sixth Period (Longest Period)
Period 6 is the longest period and
comprising of 32 elements from cesium to radon (i.e. 55Cs to 86Rn).
This period corresponds to filling up of P-shell along with N-shell, and
O-shell (containing four energy level 6s, 4f, 5d, and 6p i.e. period 6 starts
with filling up of 6s orbital and after that electron should enter 4f, but
after 6s one electron enters 5d (5d1) and after that 4f orbital
starts filling and completely filled followed by 5d and finally 6p orbital).
In 6th period, there are
8 representative elements (two s-block and six p-block elements), of the
remaining 24 elements, 10 are outer transition or d-block elements from
lanthanum (57La) and continues to
72Hf to 80Hg and 14 are f-block or inner
transition elements from 58Cerium to 71Lutetium (Lu).
For the sake of convenience, 14
f-block elements are placed at the bottom of periodic table. The 6th
period series of 14 inner transition or f-block elements that follows lanthanum
(57La) placed at the bottom of the periodic table is called
Lanthanide Series or Rare Earth Elements and it is from cerium to lutetium (58Ce
to 71Lu). In this series electrons are being added to the 4f
sub-levels.
Seventh Period (Incomplete
Period)
Period 7 is the second longest
period and it is incomplete (as to date about 109 elements have been
discovered) comprising of 26 elements (but expected to contain 32 elements)
starts from francium (89Fn). This period corresponds to filling up
of Q-shell along with O-shell and P-shell containing three energy levels 7s,
5f, 6d].
It starts with the filling up of 7s
orbital. Again after completing 7s, one electron enters 6d orbital (6d1)
then 5f starts filling up (5f1 to 5f14)
forming Actinide Series of Inner Transition Metals. After 5f14,
electrons again occupying 6d.
It contains two representative
elements (s-block), 10/8 outer transition or d-block elements and 14 inner
transition or f-block elements placed at the bottom of the periodic table
called Actinides. The 7th period series of 14 inner transition or
f-block elements that follows actinium (89Ac) in which electrons are
being added to the 5f sub-level placed at the bottom of the periodic table is
called Actinide Series and it is from thorium to lawrencium (90Th to
103Lr). In this series electrons are being added to the 5f
sub-levels.
The elements following Uranium (92U)
in 7th period with atomic number greater than 92 (Z > 92) are
known as trans-uranium elements. They are from Neptunium to Lawrencium (93Np
to 103Lw). e.g. Am, Cf, Es. All these elements do not occur in
nature and are artificially synthesized.
The elements following fermium (100Fm)
in 7th period with atomic number greater than 100 (Z>100) are
known as trans-fermium elements. Fermium is the 100th element, so
trans-fermium means beyond fermium.
Classification
of Elements based on Electronic Configuration
The elements in the periodic table has been divided into 4 blocks or groups on the basis of electronic configuration whether the last electron called differentiating electrons enters into s, p, d or f-orbitals. The periodic table has been divided into following 4 blocks on the basis of electronic configuration:
1. Representative Elements (s-block and p-block elements)
2. Noble Gases.
3. Outer transition elements (d-block Elements)
4. Inner transition elements (f-block Elements)
General Valence Shell Electronic Configuration of different Blocks of the Periodic Table
Representative Elements ---- ns1-2 np1-5
(ns1-2 np1-6)
s-Block Elements ----- ns1-2
p-Block Elements --- ns2 np1−6
d-Block Elements ---- (n–1)d1-10, ns0-2
f-Block Elements -------
(n–2)f2-14, (n–1)d0-1, ns2 OR (n–2)f1-14, (n–1)d0-1, ns2
(n–2)f0-14, (n–1)d0-2, ns2 OR (n–2)f1-14, (n–1)d0-2, ns2
1. Representative Elements
The elements of
family A of the periodic table having incomplete outermost shell are called
representative or normal or typical elements. They are so named because the
properties of these elements are represented by valence electrons. They form
group IA to VIIA and are present at left and right side of the periodic table.
Their general valence shell electronic configuration of ns1, ns2
to ns2 np5 (ns1-2 to ns2 np1-5).
They include s-block elements and p-block elements.
(a) s-block Elements
The elements
in which outer electrons enter into s-orbital having ns1-2
(i.e. ns1 to ns2) valence shell configuration
(where “n” denotes the number of outermost shell or the number of period ranges
2-7) are called s-block elements. Since last electron lies in ns orbital,
they are referred to as s-block elements.
They form group IA and IIA of periodic table found at its far
left side. There are total 13 s-block elements including hydrogen. They are highly
reactive electropositive elements with low ionization potential
showing fixed oxidation state of +1 and +2. Except Li, Be, all form ionic compound.
Their oxides are basic in nature.
(b) p-block Elements
The elements
in which outer electron enters into p-orbitals having ns2 np1-6
(i.e. ns2 np1 to ns2 np6 denotes
the number of outermost shell or the number of period ranges 2-6) valence shell
configuration are called p-block elements. Since in these elements outer
electron enters into p-orbitals (which are being progressively filled),
they are referred to as p-block elements. They from Group IIIA to Group
VIIIA of the periodic table and are located at the extreme right of the
periodic table. There are total 30 elements in six sub-groups of p-block
including noble gases except helium. Mostly they are highly electronegative
elements (non-metals). Their oxides are neutral or acidic. Mostly
they form covalent compounds.
2. d-Block Elements or Outer Transition Elements
The elements
having partially filled d-orbitals in their atoms or ions in which last
electron enters into (n – 1)d-orbitals in their atomic state or ionized state
(i.e. in their common oxidation states) are called d-block elements. Since last
electron is in the process of occupying d-orbitals, they are known as d-block
elements. In these elements, two outermost shells are incomplete i.e. in these
elements, besides the outermost valence shell (s-subshell) penultimate shell
(d-subshell) is also incomplete. These elements are called transition elements
because they show transitional (intermediate) behaviour between the two sets of
representative elements on either side of them i.e. s-block and p-block elements.
They form B-family (group IB, IIB, IIIB to VIIIB) of the periodic table and
hence are also called Group B Elements and are located in the middle of the
periodic table between s-block and p-block elements. They are characterized by
ns2, (n – 1)d1 to ns2, (n – 1)d10 OR ns2, (n – 1)d1-10
valence shell electronic configuration (where n= 4-7). They are called outer
transition elements as these elements are placed in the upper middle (outer)
portion of the periodic table. They all are metals characterized by their
variable valencies, forming coloured compounds and their ability to form
complex ions by co-ordination through co-ordinate covalent bonds.
3.f-Block Elements/Inner Transition Elements
The elements
having partially filled f-orbitals (except 71Lu, 90Th, 103Lr)
in their atoms or ions in which last electron enters into (n – 2)f-orbitals in
their atomic state or ionized state are called f-block elements. Since last
electron is in the process of occupying f-orbitals, they are known as f-block
elements. In these elements, three outermost shells are incomplete i.e. in
these elements, besides the outermost valence shell (i.e. ns-subshell) and (n –
1)d-subshell, penultimate f-subshell or (n – 2)f-orbitals are also incomplete.
Properly they should be placed after IIIB but these elements are found in a
separate position at the bottom of the periodic table. These elements are also
called the inner transition elements because the filling of electrons takes
place in the inner (n – 2)f-orbitals (4f or 5f sub-shell) i.e. two levels below
the outer (n – 1)p-orbitals (5p or 6p) and ns-orbitals (6s or 7s) orbitals
which are already filled in these elements. They are characterized by (n – 2)f0-14,
(n – 1)d0-2 , ns2 OR (n – 2)f2-14/0-14, (n –
1)d0-1/0-2 , ns2 valence shell electronic configuration
(where n = 6-7).
1. Representative Elements
Definition
The elements of sub-group A or the elements of groups 1, 2,
13, 14,15,16,17, and 18 are called Main group or Normal or Major or
Representative Elements as the properties of these elements are represented
by valence electrons.
OR
The elements in which all their inner
shells are complete but outermost shell is incomplete having less
than 8 valence electrons in their outermost shell are known as
representative elements i.e. s-block and p-block elements except inert
gases are known as representative elements. In these elements the outermost
shell gets progressively filled from group IA to group VIIIA as we move from
left to right in a period.
Reason for
Name
They are so named because the
properties of these elements are represented by valence electrons.
Group and
Position in table
They form group IA to VIIA and are present at left and right side of the periodic table.
General valence shell electronic
configuration
Their general valence shell
electronic configuration of ns1,
ns2 to ns2 np5 (ns1-2 to ns2
np1-5).
Blocks
Included
They include s-block elements and
p-block elements.
General
Characters
1. They consist of some
metals (IA and IIA Groups, some elements of IIIA group like Al, Ga, In, Tl and
miscellaneous metals like Sn, Pb, Bi, Po), all non-metals and metalloids (B,
Si, Ge, As, Sb, Se, Te).
2. There are 42 representative elements including
noble gases, of these 20 are metals
(12 s-block metals and 8 p-block metals), 14
are non-metals (N2, O2, F2, Cl2,
Br2, I2, C, P, S, Ne, Ar, Kr, Xe, Rn) and 8 are metalloids (B, Si, Ge, As, Sb,
Se, Te, At). Out of 42 normal elements, 31 elements are solids (19
metals, 4 non-metals and 8 metalloids), 2 elements are liquids (Ga and
Br2) and 9 elements are gases.
3. Some elements are
diamagnetic and some are paramagnetic. However, their compounds are generally
diamagnetic and colourless.
Sub-Division of
Representative Elements
Representative elements are of two
types namely s-block elements and p-block
elements:
(a) s-block Elements
(b) p-block Elements
(a) s-block Elements
The electropositive elements of
group IA and IIA of the periodic table in which outer electrons enter into
s-orbital having ns1-2 (i.e. ns1 to ns2)
valence shell configuration (where n = number of outermost
shell or the number of period ranges 2-7) are referred as s-block elements.
They form group IA and IIA of periodic table found at its far left side.
Since last electron lies in ns
orbital (which is being progressively filled), they are referred to as
s-block elements.
There are total 13 s-block
elements including hydrogen (there should be 14 s-block elements as He is
also associated with 1s2 electronic configuration).
They are highly reactive electropositive elements with low ionization potential showing
fixed oxidation state of +1 and +2
forming ionic compound except
Li, and Be. Their oxides are basic in nature.
Groups Included
They include
group IA and IIA. Group IA includes 6 elements namely Li, Na, K, Rb, Cs, Fr.
Francium is radioactive. The IA group is also called Lithium Family being
Lithium is the first member. Group IA elements are called Alkali Metals because
they yield strong alkalis when their oxides, hydroxides or hydrides are
dissolved in water which are completely soluble in water.
Group IIA
includes 6 elements namely Be, Mg, Ca, Sr, Ba and Ra. Radium is radioactive.
The IIA group is also called Beryllium Family being Be is the first member.
Group IIA elements are called Alkaline Earth Metals because they and their
compounds (CaCO3) are found abundance in earth crust and yield weak
bases when their oxides, hydroxides and hydrides are dissolved in water which
are sparingly soluble in water.
Electronic Configuration of s-Block Element
Alkali Metals
Since, their
valence shell configuration is ns1, so their principal oxidation
state is +1 and they form monovalent cation.
Alkaline Earth Metals
Since, their
valence shell configuration is ns2, so their principal oxidation
state is +2 and they form divalent cation.
general characters
They have following general characters:
1. Highly electropositive nature
2. Monovalent and fixed oxidation state of +1 and +2
3. Forming Ionic Bond
4. Basic nature of oxides, hydroxides and hydrides
5. Magnetic behaviour
6. Forming Colourless compounds
7. Reducing behaviour
1. They are
highly reactive
electropositive elements with low ionization potentials.
2. Group IA elements are monovalent exhibiting fixed
oxidation states of +1 while group IIA elements are divalent showing fixed oxidation state of +2.
4. Except Li,
Be, all form ionic
bonds (compound).
3. Their oxides and hydroxides are basic in nature. Group IA elements are
called alkali metals as their oxides yield strong alkalis on dissolution. Group
IIA elements are called alkaline earth metals as these metals exist as their
oxides (magnesia; MaO, lime; CaO, strontia; SrO, baryta; BaO) in the earth
which give weak alkaline solution (except BaO) on dissolution.
5. Group IA elements are paramagnetic and group IIA elements are diamagnetic. However their compounds are mostly diamagnetic.
6. They form colourless compounds except their manganates, permanganates, chromates, dichromates etc. e.g. KMnO4, NaMnO4, Na2CrO4, K2CrO4, K2MnO4 etc.
7. They are powerful
reducing agent due to their largest assize, lowest I.P and highest negative reduction potential.
8. All s-block
elements (except Be and Mg) impart characteristic colours to the non-luminous
Bunsen flame when ignited due to their low ionization enthalpies. The formation
of coloured flame is explained by the fact that these metals or their salts on
heating in a flame, the valence electrons get excited and jumps to higher energy
level, when these excited electrons return back to the original ground state,
the absorbed energy is liberated as different frequency of visible light in the
visible region of the electromagnetic spectrum and hence the flame appears
coloured. The different colours arise due to different energies required for
electronic excitation and de-excitation. The colour imparted by an element
depends upon the I.P. Higher the I.P, higher will be the frequency of radiation
absorbed and consequently lower will be frequency of radiation emitted i.e. the
colour imparted. Since Li has higher I.P, it emits radiation of lower frequency
i.e. red colour.
Be and Mg
atoms due to their small size and high I.P, their electrons more strongly bound
(because of higher effective nuclear charge) and are not excited by the energy
of the flame to higher energy states. Hence, they require high excitation
energy and therefore, these elements do not give any colour in Bunsen flame.
(b) p-block Elements
The elements in which outer electron
enters into p-orbitals having ns2 np1-6 (i.e. ns2
np1 to ns2 np6 where n denotes the number
of outermost shell or the number of period ranges 2-6) valence shell
configuration are called p-block elements. Since in these elements outer
electron enters into p-orbitals (which are being progressively filled),
they are referred to as p-block elements.
They from Group IIIA to Group
VIIIA of the periodic table and are located at the extreme right of the
periodic table. There are total 30 elements in six sub-groups of p-block
including noble gases except helium. Among these elements, 2 are liquids (Ga and Br), 9 are gases (N2, O2,
F2, Cl2, Ne, Ar, Kr, Xe, Rn) and 19 are solids. Out of 30 elements, 8 are metals (Al, Ga, In, Tl, Sn, Pb, Bi, Po), 8 are metalloids (B, Si, Ge, As, Sb, Se, Te,At) and 14 are non-metals including first
member of group IVA (C), first two members of group VA (N, P), first two
members of group VIA (O, S), first four elements of group VIIA (F, Cl, Br, I)
group.
they are mostly
highly electronegative elements (non-metals) mostly forming
covalent compounds and their oxides are usually either acidic or
neutral.
general characters
They have following general characters:
1. Highly electronegative nature
2. Polyvalent nature and variable oxidation state of
3. Forming covalent bond
4. Variable nature of oxides
5. Magnetic behaviour
6. Forming Colourless compounds
7. Oxidizing behaviour
1. They
are mostly highly electronegative
elements with high ionization potentials. They also include less active non-metals, gases, metalloids and some weak
non-active metals.
2. They
usually exhibit variable (more than one) or variety of oxidation states ranging
from – ½ +1 to +7.
3. Mostly
they form covalent
compounds.
4. Their
oxides are mostly acidic in nature (e.g. B2O3, CO2,
SiO2, NO2, P2O3, SO2, SO3,
Cl2O, Cl2O7). But
some may be neutral (CO, NO, N2O, H2O etc) or amphoteric (Al2O3,
As2O3 etc.) or basic (Bi2O3).
5. They
may be paramagnetic
or diamagnetic. However their compounds are mostly diamagnetic.
6. They
mostly form colourless compounds except NO2.
7. They
are mostly oxidizing
agent like F2,
Cl2, O2 etc. except Al, C etc.
2. The Noble Gases/Aerogens
3. d-Block Elements or Outer Transition Elements
Definition
The elements
of sub-group B or the elements of groups 3 t 12 having partially filled
d-orbitals in their atoms or ions in which last electron enters into
(n–1)d-orbitals in their atomic state or ionized state (i.e. in
their common oxidation states) are called d-block elements or outer transition
elements.
In these
elements, two outermost shells are incomplete i.e. besides the outermost
valence shell (s-subshell)
penultimate shell (d-subshell) is also incomplete.(These are the element whose
outermost s-sub-shell and
penultimate d-sub-shells are being occupied with electrons).
They all are
metals characterized by their high melting and boiling points, variable
valencies, catalytic property, forming coloured paramagnetic compounds
and their ability to form complex ions by co-ordination through
co-ordinate covalent bonds.
Reason
for calling d-Block elements
Since last
electron is in the process of occupying d-orbitals, they are known as d-block
elements.
Reason
for calling Transition elements
These
elements are called transition elements because they show transitional
(intermediate) behaviour between the two sets of representative (s and p-block)
elements on either side of them.
Reason
for calling outer Transition elements
They are
called outer transition elements as these elements are placed in the upper
middle (outer) portion of the periodic table.
Group
Formed
They form
B-family (group IB, IIB, IIIB to VIIIB) of the periodic table and hence are
also called Group B Elements and are located in the middle of the periodic
table between s-block and p-block elements.
General valence shell electronic
configuration
They are
characterized by (n–1)d1, ns0-2 to (n–1)d10, ns0-2
OR (n–1)d1-10, ns0-2 valence shell electronic configuration (where
n= 4-7).
Sub-Division
The outer transition (d-block)
elements consist of following 4
series of 10 elements
each:
Characteristics
of Transition Elements
1. Metallic Character
2. Variable nature of Bonding
3. Variable
oxidation states
4. Variable nature of oxides
5. Coloured Ions and compounds
6. Magnetic behaviour
7. Catalytic Properties
8. High melting and boiling points
9. High density and hardness
10. Complex formation
1. They all are metals are mostly less reactive characterized by partially
filled d-orbitals in their atoms or ions in ground state or in any
oxidation state (except Zn, Cd, Hg).
2. They form ionic, covalent and co-ordination (complex)
compounds as they can form all the three types of bonds i.e. ionic,
covalent, and co-ordinate bonds.
3. Excepting Zn, Cd,
Ag, Sc, Y, they show variable valency forming
more than one type of ions (e.g. Cu1+, Cu2+, Fe2+,
Fe3+ etc.). Excepting Zn, Cd, Ag, Sc, Y, they show more than one oxidation states in their
compounds. Cu shows +1 and +2.
4. They form acidic (CrO3, Mn2O7
etc), basic () and amphoteric (ZnO, Cr2O3)
oxides. Acidic nature of oxides increases with increasing oxidation state.
5. They themselves,
their ions and their compounds are highly coloured except Zn, Cd, Hg e.g.
ferric salts are brown.
6. except Zn, Cd, Hg, they all are paramagnetic
owing to presence of unpaired electrons.
7. They exhibit catalytic property and behave as catalyst in a variety of reactions.
8. Their melting points, boiling points and densities are usually very high except Sc, Y and La.
9. They form complex
ions.
Detailed
Characteristics of d-Block Elements
1. Metallic Character
They all are
metals with pronounced electropositive character. However, most of them are relatively
less reactive metals. They are hard, malleable, ductile, good conductor of
electricity and heat and form alloys.
2. Variable oxidation states
With the
exceptions of Zn, Cd, Sc, and Ag, they exhibit variety of variable oxidation
states from 0 to +8 in their compounds e.g. Cr+3 or Cr+6,
Mn+2, Mn+3, Mn+4 etc.
3. Variable nature of Bonding
They form
covalent, ionic as well as co-ordination compounds or complexes. They are
capable to form co-ordination compounds or complexes due to the presence of
small, highly charged cation with highly effective nuclear charge and
availability of vacant d-orbitals (and tendency of central transition metal to
acquire effective magic numbers of next inert gas) which can accept lone pair
of electrons donated by other groups called ligands.
4. Variable nature of oxides
They form acidic (CrO3,
Mn2O7 etc), basic () and amphoteric (ZnO, Cr2O3)
oxides. Acidic nature of oxides increases with increasing oxidation state.
2CrO3(s) + H2O ----→ 2H+ + Cr2O72-
5. High melting and boiling points
Their
melting and boiling points are generally very high except Zn, Cd and Hg due to
strong interatomic binding forces (in the form of strong covalent bonding)
between their atoms extending throughout the crystals owing to small atomic
size and unfilled d-orbitals containing unpaired valence electrons for
interaction.
6. High density and hardness
They are
hard having high densities due to their small atomic volumes (except Sc and Y).
7. Magnetic behaviour
They all are
paramagnetic due to presence of unpaired electrons with the exceptions of Zn,
Cd and Hg which are diamagnetic. Fe and Co are strongly paramagnetic and can be
magnetized and are called ferromagnetic.
8. Coloured Ions and compounds
They form
variety of highly coloured paramagnetic compounds. Colour is associated with incompletely
filled electron shells and ability for electronic transitions from one energy
level to another. Sc3+, Y3+, Zn2+, Cd2+,
Hg2+ ions are non-transitional since they either do not have
3d-electrons or ten 3d-electrons (i.e. completely filled (n-1)d-orbitals)
9. Catalytic Properties
A number of
transition metals and their compounds act as catalytic agent as they have
ability to provide low energy path for the reaction either by forming
intermediate compounds or by the change of oxidation states e.g. Cu, Fe, Ni,
Pt, Pd, W, V2O5, Cr2O3, ZnO, FeO
etc.
10. Low reactivity
They show an
increasing tendency to remain unreactive and this tendency is most pronounced
in gold and platinum. This is due to their high I.P. They are relatively more
reactive in powdered form.
4. Inner Transition Elements
Definition
The elements having partially
filled f-orbitals (except 71Lu, 90Th, 103Lr)
in their atoms or ions in which last electron enters into (n–2)f-orbitals
in their atomic state or ionized state (i.e. in their common oxidation states)
are called f-block elements or inner transition elements.
In these
elements, three outermost shells are incomplete i.e. in these elements,
besides the outermost valence shell (i.e. ns-subshell) and (n–1)d-subshell,
penultimate f-subshell or (n–2)f-orbitals are also incomplete.
Reason
for calling f-block elements
Since in
these elements, last electron is in the process of occupying penultimate f-orbitals,
they are known as f-block elements.
Group
Formed
Properly
they should be placed after IIIB but these elements are found in a separate
position at the bottom of the periodic table.
Reason
for calling Inner Transition elements
These elements
are also called the inner transition elements because the filling of electrons
takes place in the inner (n–2)f-orbitals (4f or 5f sub-shell) i.e. two levels
below the outer (n–1)p-orbitals (5p or 6p) and ns-orbitals (6s or 7s) orbitals
which are already filled in these elements.
General valence shell electronic
configuration
They have
following valence shell electronic configuration
(n–2)f2-14,
(n–1)d0-1, ns2 and
(n–2)f0-14, (n–1)d0-2, ns2
OR
(n–2)f2-14/0-14,
(n–1)d0-1/0-2, ns2 (where n = 6-7).
Sub-Division
f-block consists of two series of 14
elements each namely Lanthanide Series and Actinide Series. Lanthanides and
actinides are collectively known as inner transition elements.
The 6th period series
of 14 inner transition elements or
f-block elements that follows
Lanthanum (57La) in which electrons are being added to the 4f-orbitals placed at the bottom of the periodic table is called Lanthanide Series or Lanthanides or Rare
earth elements and it is from cerium to lutetium (58Ce to 71Lu).
The 7th
period series of 14 inner transition elements or f-block elements
that follows Actinum (89Ac) in which electrons are being
added to the 5f-orbitals placed at the bottom of the periodic table (just
beneath lanthanides series) is called Actinides Series or Actinides and
it is from Thorium to Lawrencium (90Th to 103Lr).
General Characters of f-block
elements
1. They
are metals generally paramagnetic
and form coloured ions.
2. Their
compounds are also paramagnetic.
3. The last natural
element in this series is Uranium (Z=92). Elements following uranium are called
transuranic or transuranium elements which are artificially prepared by
nuclear reactions.