1. The first organic compound prepared in the lab is:
(a) Methane
(b) Acetic acid
(c) Urea
(d) Glucose
Explanation; (c)
Urea or carbamide was the first organic compound prepared in the lab in 1828, by a German chemist Friedrich Wohler. Urea was accidentally prepared in the laboratory by controlled heating of inorganic salt, ammonium cyanate (obtained by double decomposition of ammonium chloride and potassium cyanate). This synthesis proved that there is no necessity of vital force for the preparation of organic compounds. Thus vital force theory was completely over thrown or discarded.
2. Organic chemistry deals:
(a)All hydrocarbons(b)All carbon compounds
(c)Hydrocarbons & their derivatives
(d)All of them
Explanation; (c)
The study of covalent compounds of carbon containing H, and often O, N, S, P and halogens (Cl, Br and I) containing C – C bond is termed as Organic Chemistry. It is the study of hydrocarbons and their derivatives.
Organic compounds are compounds of carbon regardless of their origin. Carbon and hydrogen form the backbone of organic chemistry. The term halogen generally excludes fluorine as fluoro-carbon compounds and their reactions differ from other halogen chemistry.
Exceptions
However there are certain compounds of carbon which are not included in organic compounds. These are carbides (CaC2), cyanides(NaCN), metal carbonates (Na2CO3), metal bicarbonates (NaHCO3), cyanates (CNO¯), sulphocyanides (CNS¯) and oxides of carbon (CO and CO2).
(ii) Lack of C – C bond
(iii) Absence of functional grope
3. Which of the following element is not present in organic compounds?
(b) N
(c) O
(d) Si
Organic compounds along with carbon and H usually contains O, N, S, P and halogens (Cl, Br and I). Si is not present in organic compounds.
(b)Ammonium sulphate
(c)Ammonium cyanate
(d)Ammonium cyanide
Urea was prepared by boiling ammonium cyanate, NH4CNO (obtained by double decomposition of ammonium chloride and potassium cyanate) with water.
(c)Natural gas
(d)All of the above
Explanation; (d)
The natural sources of organic compounds includes plants, animals, coals, petroleum, natural gas.
6. Which of the following is a type of coal?
(b) Bituminous coal
(c) Lignite (peat)
(d)All of them
Explanation; (d)
Coal is found in various forms (5 forms) which represent the different stages of conversion of vegetable matter:
7. The hardest and the driest form of coal containing 92-98% C and burns without smoke is called:
(a) Anthracite(b) Sub-bituminous coal
(c) Bituminous coal
(d) Lignite
8. The thermal decomposition of coal (bituminous) in the absence of air at 500-1000°C gives coke, coke-oven gas (coal gas) and coal tar. This process is called:
(a) Carbonization(b) Fractional distillation
(c) Destructive distillation
(d) Both (a) and (c)
9. Which one is 100% pure carbon?
(a) Coke
(b) Coal gas
(c) Gas carbon
(d) Coal tar
Explanation; (a)
Coke is the purest form of carbon comprising almost 100% carbon.
10. Coal tar is considered as “a hidden treasure” because it consists of ……… aromatic organic compounds:
(a) 215
(b) 315
(c) 415
(d) 515
Explanation; (a)
Coal tar gives more than 215 aromatics upon fractional distillation. Hence it is considered as a hidden treasure of aromatic organic compounds.
11. The unrefined petroleum is called:
(a) Crude oil
(b) Rock oil
(c) Coal gas
(d) Both (a) and (b)
Explanation; (d)
The unrefined petroleum is called crude oil or rock oil.
1. In Latin, petroleum means rock oil. It is also known as crude oil or black gold. It is found below the surface of the earth. Petroleum in the refined form is called mineral oil.
2. It is believed to be formed by the anaerobic decay (slow bichemcial and chemcial decompsotion) of buried remains of living organisms or organic matter and is trapped as large reservoirs under the impermeable or sedimentary rocks.
3. Petroleum is a naturally occurring thick viscous liquid of brown or greenish black colour with bad smell comprising of a mixture of hydrocarbons like alkanes, alkenes, cycloalkanes, aromatic hydrocarbons with small quantities of inorganic compounds of nitrogen, oxygen, and sulphur.
4. The predominate component of petroleum is alkanes. Petroleum contains alkanes upto C40.
5. Petroleum in the unrefined form is called Crude Oil or Rock Oil.
6. The crude petroleum when subjected to fractional distillation, several groups of products called petroleum products are obtained.
7. More than 500 compounds have been formed in petroleum distillate boiling below 200°C .That is why petroleum is the chief source of aliphatic compounds (hydrocarbons). It is also the main source of liquid fuels.
8. About 60% of the world reserves of petroleum are in the Middle East while 15% are in Western Hemisphere.
12. The process of a cracking takes place between temperatures:
(b) 500–700°C
(d) 500–800°C
Explanation; (d)
The optimum temperature for the process of cracking is 500–800°C.
13. Which one of the following is an addition polymer?
(b) PVA
(c) PVC
(d)All of the above
Example of Addition Polymers
14. PVC is a polymer of:
(b) Vinyl acetate
(c) Ethylene
(d) TFE
Explanation; (a)
Vinyl chloride is the monomer of Polyvinyl chloride (PVC).
(b) Polyamide
(c) Polyester
(d) None of the above
Explanation; (c)
Nylons are the synthetic fibres which contain long chain polyamide as fibre forming substance.
16. PVA is a polymer of:
(b) Vinyl acetate
(c) Ethylene
(d) TFE
Explanation; (b)
Vinyl acetate is the monomer of polyvinyl acetate.
17. The condensation polymer of hexan-1,6-dioic acid (adipic acid) and 1,6–diaminohexane is called:
(b) Terylene
(c) Bakelite
(d) PVC
Nylon is a condensation polymer of double acid dicarboxylic acid adipic acid (hexane-1,6-dioic acid, HOOC(CH2)4COOH) and double amine hexamethylene diamine (1,6-diaminohexane, H2N(CH2)6NH2). Thus nylon is a polyamide.
The same type of linkage is found in proteins.
The name nylon 6.6 is derived from the fact that both joining components contain six carbon atoms each.
It is different from natural silk in its structure but similar in appearance.
It is more elastic and finer in texture than silk.
It is used in hosiery where rayon has failed due to its inelasticity.
18.The polymer of Benz-1,4–dioic acid (terephthalic acid) and ethylene glycol is called:
(b) Terylene
(c) Bakelite
(d) PVC
Explanation; (b)
Terylene is a condensation polymer of ethylene glycol (ethane-1,2-diol) and aromatic double acid terephthalic acid (benz-1,4-dioic acid or benzene-1,4- dicarboxylic acid). [Since an acid and an alcohol react to form an ester, hence this fibre is also called polyester fibre]. Thus terylene is a polyester.
The trade name of terylene is Dacron. The same type of linkage is found in fats, oils and waxes.
It is produced by the condensation polymerization of ester formed by the esterification of terephthalic acid and ethylene glycol.
It is more resistant to water and has better sweat absorbing quality than nylon.
Its fabrics have more strength, resistant to heat, better texture and tailoring qualities.
19. The polymer of formaldehyde and phenol is called:
(a) Nylon
(b) Terylene
(c) Bakelite
(d) PVC
Explanation; (c)
In presence of dilute base, phenol reacts with formaldehyde to produce a mixture of ortho and para-methylol phenols which are polymerized into a condensation polymer, Bakelite (first synthetic plastic), a thermo-setting plastic, once moulded, it cannot be remoulded and remelted.
20. Which one of the following is a type of isomerism?
(b) Stereo isomerism
(c) Metamerism
(d) All of the above
Explanation; (d)
21. An acceptor of pair of electron is termed as
(a) Nucleophile
(b) Electrophile
(c) Carboanion
(d)Anion
Explanation; (b)
An Electrophile is an electron pair acceptor during a reaction. They are electron-deficient species with vacant orbital usually have positive charge.
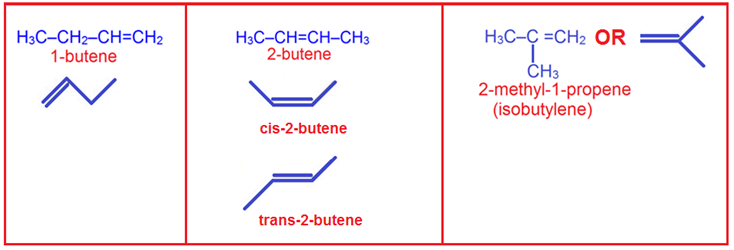
22. Butene with molecular formula C4H8 has ________ isomers.
(b) 3
(c) 4
(d) 6
Explanation; (c)
C4H8, an alkene can show geometrical isomerism, functional isomerism and structural isomerism. There are 3 possible structural isomers. They are But-1-ene, But-2-ene, 2-methylpropene.
2-Butene is the simplest alkene exhibiting cis/trans-isomerism (also known as (E/Z)-isomerism); that is, it exists as two geometric isomers cis-2-butene ((Z)-2-butene) and trans-2-butene ((E)-2-butene). Geometric isomerism (also known as cis-trans isomerism or E-Z isomerism) is a form of stereoisomerism. Thus there are total 4 possible isomers of C4H8.
23. Decane has ________ isomers:
(b) 9
(c) 35
(d) 75
Explanation; (d)
Decane has 75 isomers.
No. of Possible Isomers of Alkane
24. A nucleophile is a
(b) Arrhenius acid
(c) Lewis acid
(d) Arrhenius base
Explanation; (a)
A nucleophile is a chemical reagent which can donate an electron pair in a reaction to an electron deficient part to make a new covalent bond is called a nucleophile. They attack on the positive site of the substrate molecule or loves electron. Thus they act as an electron pair donor during a reaction. They are represented by N‾ or ![]() or Nu‾. They are also called Lewis Base as they are electron-rich species and hence have an affinity for positive nucleus.
or Nu‾. They are also called Lewis Base as they are electron-rich species and hence have an affinity for positive nucleus.
The name nucleophile means “nucleus-loving” which indicates that a nucleophile always attacks regions of low electron density (positive centers) in the substrate molecule due to sufficiency of electrons.
All nucleophiles are either negative ions or anions having negative charge (even partial) or neutral molecules with p-electrons or unshared or lone electron pair. All anions, unsaturated compounds (alkenes, alkynes, arenas) and compounds having lone pair of electrons are considered to be nucleophile.
25. Which molecule has longest carbon chain?
(b) Neopentane
(c) Neohexane
(d) n-pentane
Explanation; (d)
n-pentane has the longest carbon chain of 5.
Iso-pentane has the carbon chian of 4.
Neopentane has the carbon chain of 3.
Neohexane has the carbon chain of 4.
26. Which molecule all the least C-C distance?
(a) C2H6
(b) C2H4
(c) C2H2
(d) C4H8
Explanation; (c)
C–C bond distance is least in compound containing C≡C triple bond. C2H2 is ethyne containing C–C triple bond.
27. What is the value of C–C bond length in ethyne?
(a) 154 pm
(b) 139 pm
(c) 134 pm
(d) 120 pm
Explanation; (d)
C–C bond length in ethyne is least (120 pm) as it contains carbon carbon triple bond.
28. As number of C atom in homologous series increases, then which of the following will decrease?
(a) B.P/M.P
(b) Solubility
(c) Density
(d) Both a and c
Explanation; (b)
Solubility decreases with increasing molecular mass.
29.Which of the following is the first member of ester homologous series?
(b) Methyl ethanoate
(c) Methyl methanoate
(d) Ethyl methanoate
Explanation; (c)
Methyl methanoate is the first member of ester series.
34. How many isomeric alcohols can be obtained from C4H9OH?
Explanation; (c)
C4H9OH is butyl alcohol containing butyl radical which has 4 isomeric forms, so this alcohol also has 4 isomers.
35. How many esters are there with the molecular formula C4H8O2?
Explanation; (d)
Since molecular formula, C4H8O2 obeys general formula CnH2nO2, so possible isomers should belong to carboxyl acids and esters. There are 4 isomers esters possible for the molecular formula C4H8O2
36. Sodium or potassium salts of aryl or alkyl sulphonated acids are called:
37. The general formula for ethers is:
38. The most productive distillate of coal is
39. In Bucky balls the smallest molecule known contains ……….. carbon atoms
40. All elements given below can be detected in an organic compound without using Lassaigne’s solution except
41. When lime water turns milky, it shows the presence of …………..in the organic compound.
42. Which observation confirms sulphur in the organic compound when Lassaigne’s solution is reacted with specific reagent?
43. Lassaigne’s solution is reacted with reagents like HNO3 and AgNO3 to detect ………. in the organic compound
Explanation; (c)
In secondary alcohols, - OH group is present at carbon number 2. Hence sec-butyl alcohol is called butan-2-ol.
45. The IUPAC name for H2C=CH – CH = CH2 is
46. Which one is ethonoic anhydride?
An Acid anhydride can be defined as a non-metal oxide which forms an acidic solution when reacted with water. The non-metals which are capable of reacting with water are only called Acid anhydrides and non-metals that do not react with water are not acids anhydrides.
So we can conclude that all non-metals are not acid anhydride; those non-metals that react with water from acid are only acid anhydride. For example, carbon monoxide is not an acid anhydride, though it is an oxide of carbon because it does not react with water.
In organic chemistry, acid anhydride is a functional group consisting of 2 acyl groups combined by an oxygen atom. In the case of organic chemistry, acid anhydrides are formed from the dehydration of two carboxylic acid groups. Alkanoic anhydride/carboxylic anhydride are formulated as (RCO)2O.
47. The structural formula of methyl ethanonate is
(a) CH3 – COO – CH3
(b) CH3 – OOC – CH3
(c) CH3 – CO – OH
(d) Both a and b
Explanation; (d)
48. The IUPAC name of H – CO – NH2 is
(a) Formamide
(b) Methanamide
(c) Acetamide
(d) Ethanamide
Explanation; (b)
The IUPAC name of containing amide group is alkanamide. The common name of amide is carboxamide.
49. Propanoyl chloride is a member of
(a) Acid amides
(b) Acid halides
(c) Acid anhydride
(d) Alkyl halides
Explanation; (b)
Propanoyl chloride is a member of Acid halides.
50. Which one is organic compound?
(a) KCN
(b) CS2
(c) NH4OCN
(d) None of these
Explanation; (d)
Metallic cyanides, cyanates, thiocynates, carbonates, bicarbonates, carbides, dicarbides, oxides of carbon like CO, CO2, C3O2, and carbon disulphide are inorganic compounds. All given compounds are inorganic compounds.
51. Lassaigne’s solution is also called
(a) Bromine water
(b) Sodium extract
(c) Copper extract
(d) Nickel extract
Explanation; (b)
Lassaigne’s solution is also called Sodium extract.
52. Which element is not detected in organic compound?
(a) Carbon
(b) Hydrogen
(c) Oxygen
(d) Nitrogen
Explanation; (c)
Oxygen cannot be detected in organic compounds directly.
53. A liquid alkane may be converted into gaseous hydrocarbon by
(a) Oxidation
(b) Reduction
(c) Cracking
(d) Hydrolysis
Explanation; (c)
Cracking is used to covert liquid alkane of long chain hydrocarbon to gaseous hydrocarbon of smaller chain. Lower alkanes are gaseous in nature while higher alkanes are either liquids or solids.
54. We use (C2H5)4Pb in the gasoline to reduce
(a) Consumption of fuel
(b) Price of fuel
(c) Octane number of fuel
(d) Knocking of engine
Explanation; (d)
(C2H5)4Pb is an anti-knock agent that reduces of knocking of engine.
55. When n-octane is heated in the absence of air at high temperature in the presence of catalyst, it changes to 2,2,4-trimethylpentane. This process is called
(a) Polymerization
(b) Condensation
(c) Reforming
(d) Cracking
Explanation; (c)
Reforming is the conversion of normal-alkane or straight chain alkanes into isomeric branched chain alkanes.
56. Which of the following is cycloalkane?
(a) C6H14
(b) C6H12
(c) C6H10
(d) C6H8
Explanation; (b)
Cycloalkanes have general formula CnH2n (n=3-infinity). Here C6H12 obeys the general formula.
57. Which isomers have difference in both their physical and chemical properties?
(a) Skeletal isomers
(b) Positional isomers
(c) Metamers
(d) Functional isomers
Explanation; (d)
Functional isomers have different chemical properties due to different functional groups. Functional isomers also have different physical properties.
58. The isomerism exhibited by C5H11OH is
(a) Positional isomerism
(b) Functional isomerism
(c) Chain isomerism
(d) All of these
Explanation; (d)
The molecular formula C5H11OH belongs to alcohol which can show all the three types of isomerism.
59. Which isomerism is NOT shown by alkene?
(a) Chain isomerism
(b) Metamerism
(c) Geometrical isomerism
(d) Position isomerism
Explanation; (b)
Alkenes can show chain isomerism (different longest chain but same formula), position isomerism (substituents at different places) and geometrical isomerism (cis-trans) but not metamerism, because it does not contain any functional group like two alkyl groups on either side (R−O−R′).
Metamerism is due to presence of different alkyl groups attached to the same polyvalent functional group or atom. This kind of isomerism is not possible in case of alkenes.
60. Which type of isomerism is being exhibited by FCH=CHF?
(a) Chain isomerism
(b) Structural isomerism
(c) Geometrical isomerism
(d) Position isomerism
Explanation; (c)
FCH=CHF has double bond in which each carbon is surrounded by two different groups. All the two conditions allow this compound to show geometrical isomerism.
(b)
(c)
(d)