Substance or Pure Substance
1. A piece of matter in pure form is called a substance. A sample of pure matter whose composition is uniform throughout is called a substance2. It has a fixed composition and specific properties.
3. It is a type of substance which cannot be separated into more than one type of components by physical methods having same properties throughout their bulk.
4. Every substance has physical and chemical properties.
5. They are made up of one kind of matter.
6. Elements and compounds are the examples of pure substances.
Examples
Tin, sulphur, diamond, water, pure sugar (sucrose), table salt (sodium chloride), baking soda (sodium bicarbonate) etc.
Impure Substances
It is a type of substance which can be separated into their components by physical methods.
Examples
The only example of impure substance is mixtures.
Atomic Number (Z) = number of Protons (P) = Number of electrons (e)
The sum of number of protons and neutrons in the nucleus of
an atom is called Mass Number or Nucleon Number denoted as “A”.
OR
the total number of "nucleons" (Protons and Neutrons)
in the nucleus of an atom is called mass number. (protons and neutrons are
collectively called nucleons).
e.g. Mass number of Na is 23 because its nucleus contains 11
protons and 12 neutrons.
Mass Number (A) = Atomic Number (Z) + number of neutrons (n)
And
No of neutrons = Mass number (A) – atomic number (Z)
Representation
Mass number is written as superscript on the left hand side of the chemical symbol of element. e.g. 12C, 14N
Calculating PEN (Protons-Electrons-Neutrons) Numbers
The identical atoms with same atomic number unite to form an element and different elements combine together to form compounds. Therefore, elements are the simplest substances that we can use and investigate in chemistry because an element cannot be split into other substances (unlike compounds).
An element is a pure substance made up of only one type of atoms (unlike compounds) which cannot be further divided (split) into simpler substances by ordinary chemical means in which all the atoms are chemically identical having same atomic number. For example; Gold is an element and if it is broken into small pieces, each piece will retain the properties of gold.
An element is a pure substance made up of same type of atoms with same atomic number and cannot be decomposed into simpler substances by ordinary chemical reactions.
Elements may exist as atoms
like the Noble Gases e.g. helium He or as molecules e.g. hydrogen H2 or
sulphur S8.
Examples of some elements
1. Gaseous Elements ; Hydrogen, Oxygen, Nitrogen, Fluorine, Chlorine, Helium, Neon, Argon, Kr, Xe, Rn etc.2. Liquid Elements; Bromine, Mercury.
3. Solid Elements; All metallic elements (e.g. Na, K, Al) & some non-metals (C, S, I, P)
Natural Abundance of Elements in Human Body
► Most useable metal is Fe.
► Most reactive metal is Cs
► The lightest metal is Li
► The heaviest metal is Os
► Most malleable, ductile metals are gold, and silver.
Metals have been
subdivided into:
1.
Normal or representative
metals
2.
Transition metals
Physical and
Chemical Properties of Metals
Physical properties
1. Physical
State
2. Hardness
3. High density
4. Metallic luster
5. opaque nature
6. High tensile strength
7. Malleable
8. ductile
9. High m.p & b.p.
10. High conductivity
11.
Magnetic behaviour
12. Alloy formation
13. Position in periodic
table
14. Total number
1.
Solid Physical State
All metals are solid at room temperature except Hg, Cs and Ga.
2.
Hardness
They are hard except Na and K which are soft and can be cut with a knife.
3. High density
They have high density and usually more denser than water except Li, Na,
K.
4. Metallic lustre
They have characteristic shiny metallic lustre (shine) on their surface.
(especially when cut).
5. opaque nature
They are opaque (light cannot pass through them).
6. High tensile strength
They have high tensile strength i.e. they are tough and strong.
7. Malleable
They are malleable (stretchable or dentable) i.e. hammered into sheets
8. ductile
They are ductile (flexible) i.e. hammered into sheets
9. High m.p & b.p.
They have high melting and boiling points.
10. High conductivity
They are good conductor of heat and electricity.
11.
Magnetic behaviour
Most
of the metals are paramagnetic i.e. attracted in a magnetic field.
12. Alloy formation
Metals form alloys when mixes with each other.
13. Position in periodic table
Chemical properties of Metals
1. Forming Positive ions2. Metallic Bonding
3. Fewer no. of valence electrons
4. Positive Oxidation state
5. Low ionization energy
6. Reducing agent
7. Basic Nature of oxide
8. Basic Nature of hydrides
9. Action of water
10. Action of dilute acids
11. Action of alkalis
12. Action of air
1.
Forming Positive ions
Metals are electropositive
elements and thus they act as electron donor and readily form positive ions by losing their valence electrons
typically attaining noble gas electronic configuration due to their low ionization energy.
2.
Metallic Bonding
Metals atoms or ions are held together by metallic
bonding.
3.
No. of valence electron
Most of the metals have less than 4
valence electrons except some transition metals which may
have more than 4 valence electrons (many metals have only one or two valence
electrons).
4.
Oxidation state
They always exhibit positive oxidation
state ranging from +1 to +7 (may be zero or
even fractional & +8 for Os and Ru).
5.
Low ionization energy
They have low I.P. values due to their large atomic size and less nuclear charge which lead to their strong electropositive character.
6. Reducing agent
They are always reducing agent.
7.
Basic Nature of oxide
They mostly form basic oxides e.g. Na2O, Li2O, CaO, MgO, BaO, Na2O2,
etc. except some transition metal oxides which may form either form acidic (CrO3,
Mn2O7 etc.) or amphoteric (ZnO, Cr2O3)
or some normal metals oxides (BeO, Al2O3, PbO, PbO2,
SnO, SnO2).
Na2O
+ H2O -------> 2NaOH
8.
Basic Nature of hydrides
They
form mostly stable basic hydrides except transition metals which form interstitial hydrides.
9.
Action of water
Many metals dissolve chemically in water at different temperature
evolving H2 gas. Iron, Zinc, magnesium react only with steam to
produce respective oxide and H2 gas while all other metals react
with cold water producing corresponding alkali liberating H2 gas.
10.
Action of dilute acids
Dilute acids dissolve most of the metals (except Cu, Ag, Au, Pt, Pb etc.)
to produce salt and H2 gas.
M(s) + 2H+ --------> M2+(aq) + H2
11.
Action of alkalis
Most of the metals are unaffected by
alkalis. Amphoteric metals like Al, Zn, Sn etc.
dissolves in alkalis forming their respective oxysalt evolving H2
gas.
Zn(s) + 2NaOH --------> Na2ZnO2aq) + H2
12. Action of air
Most
of the metals corrode in air giving their respective oxides.
Definition
Examples of
Non-Metals
1. Gases; H, O, N, F, Cl, He, Ne, Ar, Kr, Xe, Rn
2. Liquid; Br
3. Solids; C, P, S, Se, I
Physical properties of Non-metals
1. Occurrence in all three Physical State
2. Hardness
3. Low density
4. Lack of Metallic luster
5. Opaque nature
6. Low tensile strength
7. Non-malleable
8. Non-ductile
9. Low m.p & b.p.
11. Non-magnetic behaviour
12. Position in periodic table
13. Alloy formation
14. Total number
1.
Physical State
They
are found in all the three states of
matter.
2.
Hardness
Solid
non-metals are soft and brittle except diamond (hardest natural element known).
3.
Low density
They
have low density and are lighter than metals. However all of them are more
denser than water.
4.
Lack of Metallic luster
They
lack metallic luster and usually they are dull except diamond, graphite, Si and iodine.
5. opaque nature
Solid non-metals are opaque. However gaseous non-metals are transparent and light can pass through
them.
6.
Low tensile strength
They
low high tensile strength
7.
Non-malleable
They
are brittle and thus non-malleable i.e. cannot be hammered into
sheets.
8.
Non-ductile
They are non-ductile i.e. cannot be hammered
into wires.
9. Low m.p & b.p.
They
have low melting and boiling points except carbon (3350°C).
10.
Low conductivity
They
are poor conductor of heat and electricity except graphite (super conductor).
11.
Non-magnetic behaviour
Most
of the non-metals are non-magnetic.
12.
Position in periodic table
13.
Alloy formation
They do not form alloys with each other. However some non-metals like C,
P, Si form alloys with metals.
14.
Total number
Chemical properties of Non-metals
1. Forming Positive ions2. Bonding
3. Greater no. of valence electron
4. Showing negative and positive Oxidation state
5. High Electron affinity
6. Oxidizing agent
7. Variable Nature of oxide
8. Variable Nature of hydrides
9. Action of water
10. Action of dilute acids
11. Action of air
12. Action of alkalis
1.
Forming Positive ions
Non-metals are electronegative elements (except 6 noble gases) and thus
they act as electron acceptor and readily form negative ions by gaining one or
more electrons typically acquiring next
noble gas electronic configuration of the same period due to their large
negative electron affinities.
2.
Bonding
Non-metals
atoms or molecules are held together either by covalent bonds or van der Waal’s
forces.
3.
Greater No. of valence electron
Most of the non-metals have more than 3 valence electrons (except He
which has only 2) ranging from 4 to 7 (except all noble gases having 8 except
He).
4.
Oxidation state
They exhibit variety of oxidation states (which may be negative,
positive, zero or even fractional) ranging from -1/2 to +7.
5.
High Electron affinity
They have high electron affinity values due to their small atomic size
and greater nuclear charge which lead to their strong electronegative
character.
6.
Oxidizing agent
They
are always oxidizing agent (except hydrogen and carbon).
7.
Acidic Nature of oxide
They mostly form acidic oxides. However some non-metallic oxides may be
neutral (CO, NO, N2O, H2O).
CO2
+ H2O --------> H2CO3
SO2
+ H2O --------> H2SO3
8. Variable Nature of hydrides
Their
hydrides are either neutral (CH4), basic (NH3) or acidic
(HF, HCl, HBr)
9.
Action of water
Non-metals in general do not react with cold or hot water. However, red
hot carbon reacts with steam at elevated temperature to form water gas.
C + H2O --------> CO
+ H2
10.
Action of dilute acids
Non-metals
are generally inert toward dilute acids.
11.
Action of air
Non-metals are generally not affected by cold-dry air. However, when they
ignited in air, they react with oxygen of air forming respective oxides which
are acidic in nature.
12.
Action of alkalis
Non-metals
are generally inert toward alkalis
1.8 Metalloids or Semi-metals
Examples of
Metalloids
There are total 8 metalloids:
1. Boron of group IIIA
2. Silicon and germanium of group IVA
3. Arsenic and antimony of group VA
4. Tellurium and polonium of group VIA
5. Astatine of group VIIA
3. The symbols of some elements are derived from their Latin name. E.g.
Explanation
1.In the
formation of a compound, there is always a chemical change between the
components element, so that the compound formed is a new substance.
2.In
a compound, the constituent elements lose their characteristic properties.
Thus a compound always possesses properties entirely different from those of
their constituent elements. e.g.
(i) Zinc
is a grey solid and sulphur is yellow solid while their compound zinc sulphide
is white.
(ii) Carbon dioxide (CO2) is a
compound which neither burns nor helps in burning, while its constituent carbon
itself burns and oxygen helps in burning.
3. The compound is formed by the fixed ratio of atoms of
component elements, e.g. water is a compound of hydrogen and oxygen is which H
and O are present in the ratio of 2:1 by atoms.
4. The melting and boiling points of compounds
are sharp.
Examples
Sodium Hydroxide (NaOH)
Hydrochloric Acid (HCl)
Sodium Chloride (NaCl)
Methane (CH4)
Calcium Carbonate (CaCO3)
Types of Compound According to bonding
Covalent or Molecular compounds; comprising of molecules in which atoms are covalently bonded.
Ionic or Electrovalent compounds; comprising of aggregate of cations and anions in crystal lattice
Types of Compound According to Origin
Inorganic compounds; Compounds of all elements except carbon, also contain C in special forms
Organic compounds; Covalent compounds of carbon , mostly hydrocarbons and their derivatives
Types of Compound According to Taste
1. Acids; Containing ionizable H+ ions. e.g HCl, HBr, HI, HNO3, H2SO4, CH3COOH etc.
2. Bases; Containing ionizable OH- ions. e.g. NaOH, KOH, Ca(OH)2, Ba(OH)2 etc.
3. Salts; Acid-base neutralization product. e.g. NaCl, KCl, NaBr, NaI, KBr, KI etc.
Types of Compound According to Number
of elements
1.Binary compounds; comprising of only 2 different elements.
2. Ternary compounds; comprising of three or more elements.
Types of Compound According to
Solubility
1. Soluble compounds
2. Sparingly soluble compounds
3. Insoluble compounds
Types of Compound According to
Conductivity
1. Electrolytes
2. Non-electrolytes
1.11 Mixtures
Types of Mixtures
There are two main types of mixtures
1.Homogenous Mixtures
1.Mixtures having uniform composition are called homogenous mixtures.
2. In a homogenous mixture all the substances are evenly distributed throughout the mixture. Their components cannot be seen with naked eyes.
3. They are also known as solutions or alloys.
e.g.
air, aqueous sugar solution etc.
2. Heterogeneous Mixtures
1. Mixtures which do not have uniform composition throughout their mass are called Heterogeneous Mixtures.
2. In a heterogeneous mixture the substances are not evenly distributed.
e.g.
soil, rocks, ice cream, chocolate chip cookies, pizza, rocks etc.

Examples
1.12 Valency
Old Definition
Valency of
an element is defined as the number which expresses the combining or displacing
tendency of an element with other elements”. “Valency may also be defined as
the number of hydrogen atoms which combine with or displace one atom of an
element”. Valency is a simple whole number.
For Example
valency of chlorine is one as it
combines with one H atom to form HCl and valency of oxygen is two as it
combines with two H atoms to form H2O.
Modern Definitions
Valency is defined as the number of electrons lost
or gained by an atom of the element during a chemical reaction in order to
complete its outermost shell (Octet)”.
For example
valency
of calcium is 2 because it loses two electrons to form Ca2+
ion. Similarly valency of oxygen is also
2 as it accepts two electrons to form O2‒ ion.
Hund’s Rule Definitions
The number of unpaired electrons or partially filled orbitals constitutes the valency of an element.
For example;
Nitrogen has 3 unpaired electrons in
its valence shell, so its valency is 3.
Variable Valency
Some elements show more than one type of valency these types of valency are called variable valency. These types of compounds show a valency in one compound and another valency in other compounds. Many elements show variable valency. Variable valency is shown by elements like Iron, mercury, and copper. Transition elements show variable valency. The valency of iron may be 2 or 3 and that of copper may be 1 or 2.
The elements having variable
valencies are shown below:
The element that exhibits
lower valency will be suffixed with “ous”. While the element that exhibits
higher valency will be suffixed with “ic”.
Significance
The
formula of compounds can be found out by means of valencies of two combining
atoms.
Explanation
1.Valency is simply a whole number
without positive or negative sign.
2.The valency of elements ranges 1 to
7. Valency of an element cannot exceed
7.
3.Valency of an element cannot be zero
except noble gases.
4.Valency of an element cannot be in
fraction.
Types of elements on the basis of valency
Determination of Valency of X
in compound
Determination of Valency of M
in compound
Types of Valency
There are two
types of valency:
1. Electrovalency
2. Covalency
Electrovalency
In the formation of an ionic or electrovalent compound, the number of electrons lost or gained by one atom of an element to achieve the nearest inert gas electronic configuration is known as its electrovalency.
For example electrovalency of Na is 1 as it loses one electron. Electrovalency of Mg is 2 as it donates 2 electrons.
Covalency
In the formation
of a covalent compound, the number of electrons shared by one atom of an
element to achieve the nearest inert gas electronic configuration is known as its
covalency. If an atom shares 1 electron, its covalency will be 1.
1.13 Ion or Simple Radical or Radical
Definition
The electrically charged atoms or
group of atoms formed by the loss or gain of electrons are called Ions. An
atom or group of atoms that carries an electric charge which is formed by the loss
or gain of one or more electrons is called an ion.
The electrically charged
atoms are called Ions. An atom or group of atoms that carries an electric
charge either positive or negative behaving as an entity which is formed by the
loss or gain of one or more electrons is called an ion. This loss or gain of
electrons takes place to obtain a full outer shell of electrons.
e.g. Na+, NH4+, Cl¯, CO32¯.
Characteristics
1. Ions are not electrically neutral as in ion, number of protons and
electrons are not equal. Thus an ion is electrically charged because it
contains different number of positively charged particles (protons) and
negatively charged particles (electrons).
2. Ions always exist in ionic compounds
only.
3. Ions usually possess complete octet. For instance Na+
or Cl- both contains 8 electrons in their valence shell. The ions
formed by normal elements have complete octet while ions formed by transition metals have incomplete octet
(except Sc3+, Y3+, Ti4+, Cr6+, V5+,
W6+ etc.).
4. The
electronic structure of ions of elements in Groups I, II,III, VI and VII will
be the same as that of a noble gas (e.g. helium, neon, argon, krypton, xenon
and radon).
5. All
metals lose electrons to other atoms to become positively
charged ions called cations.
6. All
non-metals gain electrons from other atoms to become negatively
charged ions called anions.
7. When writing about ions, we use the
notation 1-, 2+ etc. to describe the charge of the ion, with the number
first followed by the sign (+/-). It is incorrect to write them the other
way around like +1, -2 etc. as this refers to the oxidation state, not the
charge.
8. group I (Li, Na, K): form 1+ ions, group II (Mg, Ca, Ba): form 2+ ions,
group III: form 3+ ions
9. group V (N, P, As): form 3- ions, group VI (O, S, Se): form 2- ions, group VII: form 1- ions
10. ions
from common oxyacids: NO3‒ (nitric acid), SO42‒
(sulfuric acid)
Naming Monoatomic
Ions
1. To name monoatomic
or single element positive ions of representative elements of group A
like Na+, K+, Mg2+, etc. , write the name as
from the periodic table adding the word ion afterwards.
2. To name monoatomic or single element negative ions like F–, O2–, P3– etc., write the name from the periodic table replacing the ending with ide adding the word ion after the name.
3. To name monoatomic or single element positive ions of transition elements like Fe3+, Cu2+, Co3+ etc., write the ionic charge (1+, 2+, 3+ etc.) as a Roman Numeral in parenthesis. So Cu2+ would be the copper(II) ion. Fe3+ would be called iron(III) ion
4. Some monoatomic
positive ions of non-transition elements or representative elements like
Pb, Sn, Sb, As are also named by writing their ionic charge as Roman Numeral in
parenthesis.
Types of Ions based on complexity
1. Simple Ion or simple radical or Monoatomic Ion; Na+, Cl–.
2. Compound ion (Polyatomic ions); NH4+,
HCO3–.
3. Complex ion or complex radical; [Fe(CN)
6] 4¯,[Cu(NH3)4]2+
4. Molecular Ions; [NO+,
CO+]
Types of Ions according to
Charge
There are two types of ions, cations and anions.
(a) Cations or
metallic ions or acid radicals, formed by loss of election e.g. Na+
(b) Anions or nonmetallic or basic
radicals, formed by gain of electron e.g. Cl–
1.14 Cation or Basic Radicals or Metallic Radicals
1 The positively charged ion formed by the loss of electron by neutral metal atom containing more protonsthan electrons is called Cation. Loss or removal of electron from neutral metal atom gives cation. e.g.
Na → Na+ + 1e–
Mg → Mg2+ + 2e–
Al → Al3+ + 3e–
2. The size of cation is smaller than its parent atom.
3. They are called cations as they move towards cathode (negative electrode) during electrolysis.
4. They are called basic radicals as they are originated from bases.
5. They are mostly metallic in nature except cationic non-metallic radicals like NH4+, PH4+, NO2+, etc.
1.15 Anion or Acid Radicals or Non-metallic Radicals
1. The negatively
charged ion formed by the gain of electron by neutral non-metallic atom
containing more electrons than protons is called Anion. Gain of electron by
neutral atom gives anion. e.g.
Cl + 1e– → Cl–
O + 2e– → O2–
N + 3e– → N3–
C + 4e– → C4–
2. The size of anion is larger than its parent atom.
3.They are called anions as they move towards anode (positive electrode) during electrolysis.
4. They are called acidic radicals as they are originated from acids.
5. They are always non-metallic in
nature. Most of the anions contain oxygen and they are called oxyanions.
1.16 Formula Unit
Ionic compounds do not exist
as individual discrete molecules. In some
crystalline compounds (NaCl, KCl, CaF2 etc.) and in some covalent
network solids, there are no discrete molecules but the atoms are bounded to
one another in a network structure as aggregate of positive and negative
ions. Such compounds are represented by their simplest or empirical
formula which simply shows the relative number of atoms of each component.
Formula unit is
the lowest whole number ratio of ions in an ionic compound. It expresses the
smallest collection of oppositely charged ions that would be neutral in an
ionic compound or an ionic crystal lattice i.e. it is the lowest ratio of ions
represented in an ionic compound.
[Note; the formula unit is analogous to molecule in a molecular
compounds].
A formula unit in chemistry is the empirical
formula of an ionic or covalent network
solid compound used as an independent entity for stoichiometric calculations. A
formula unit shows the kinds and numbers of atoms in the smallest representative unit of a
substance.
Examples include
ionic compounds like NaCl and K2O and covalent networks compounds
such as SiO2 and C (as diamond or graphite).
A formula unit is
electrically neutral as it contains oppositely charged ions (cations and
anions) in lowest possible whole-number ratio so that the sum of charges of
ions becomes zero.
1.17 Molecular ions
when a molecule loses or gains electrons is called molecular ions. For example CH4+
Molecular ions also possess positive or negative charge like any ion.
If Molecular ion has negative charge it is known as anionic
molecular ion, if it has positive charge then it is known as cationic molecular
ion.
1.18 Free Radicals
Free radicals are atoms and group of atoms having number of
unpaired electrons. It is represented by putting a dot over the symbol of an
element.
For example: Ho, Clo,
Free radicals are formed when homolytic breakage of bond between two atoms takes place by the absorption of heat or light energy.
Free radical is very reactive chemical species.
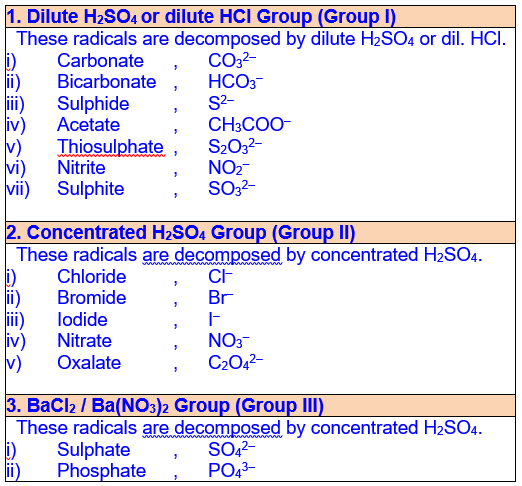
1.19 List of Acid Radicals (Anions)
in Salt Analysis
Acid radicals (Anions) are grouped on the basis of their decomposition either by dilute or concentrated H2SO4 and are identified by performing DRY TEST.
1.20 List of Basic Radicals (Cations) in Salt Analysis
Basic radicals (Cations) are
grouped on the basis of their precipitation by different reagents in the
increasing order of Ksp of their salts.
1.21 Separation of Basic
Radicals (Cations)







































.jpg)