2. Oxidation number change method
3. Aggregate redox species method (or ARS method) Jette and LaMev developed the method for balancing redox-reactions by ion electron method in 1927.
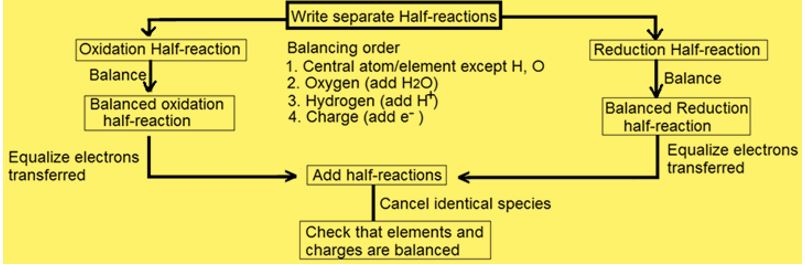
1. Convert molecular equation into ionic equation.
2. Split ionic equation into two half-reactions or equations (one for the species that is oxidized and its products and for the species that is reduced and its products).
3. Balance the atoms other than O and H by inserting suitable coefficients in each half reaction separately.
4. (a). For reactions in an acidic medium, add one H2O for each O atom to balance O and one H+ for each H atom to balance H. The O atoms are balanced first.
(b) For reactions in a basic medium, to balance O atom add two OH– per O
atom to the side that
is deficient in O while add one H2O on the other side at the same time. To balance H
atom, add one H2O per H atom to the side
where H is less while add one OH– on the other side at the same time.
OR
For reactions in a basic medium, balance O and H atoms as balanced in acidic medium. Then add OH– ions to both sides of the equation equal to the number of H+ ions. (If there is no H+ then do not add OH– ions). Where H+ and OH– appear on the same side of the equation, combine the ions to give H2O.
5. Balance the charge in each half equation by inserting e– (electrons) as a reactant or product.
6. Equalize the loss and gain of electrons in both half equations by multiplying one or both half reactions by appropriate co-efficients.
7. Add the two half equations after canceling
electrons. If H+, OH– or H2O
appears on both sides of the final equation, cancel
out the duplications.
(To verify equation is atomically and electrically balanced, count net charges on both sides of the equation, if charges are same then equation is balanced).
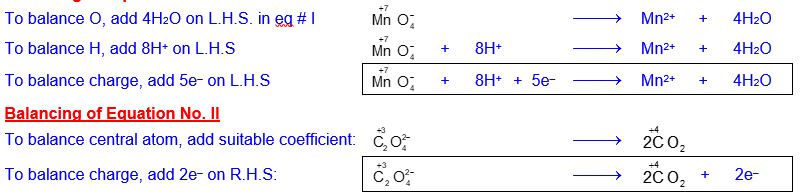
Balance the following equation in acidic medium by I.E.M.
Solution
Splitting
of ionic equation into two half-reactions
After writing oxidation number of those species that undergo any change,
split ionic equation into two half reactions:
Overall Redox Reaction
To balance e–, oxidation half-equation is multiplied by 5 (so that 10e– are produced) and the reduction half-equation is multiplied by 2 (so that the same 10e– are consumed) and then add resultant equations after canceling e–.
Solution
First molecular equation splits into ionic form and then oxidation number
of only those species have been written who have undergone any change (the
species that do not undergo any change in oxidation no. do not appear in
equation).
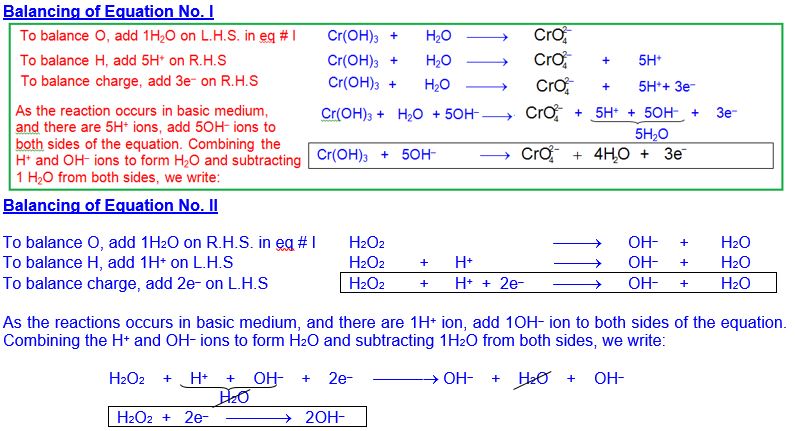
Balance the following equation in basic medium by
I.E.M
Splitting of ionic equation into two half-reactions
After writing oxidation number of those species that undergo any change, split ionic equation into two half reaction:
To equalize the number of electrons, eq. I is multiplied by 2 and eq. II by 3 and then resultant equations are added.
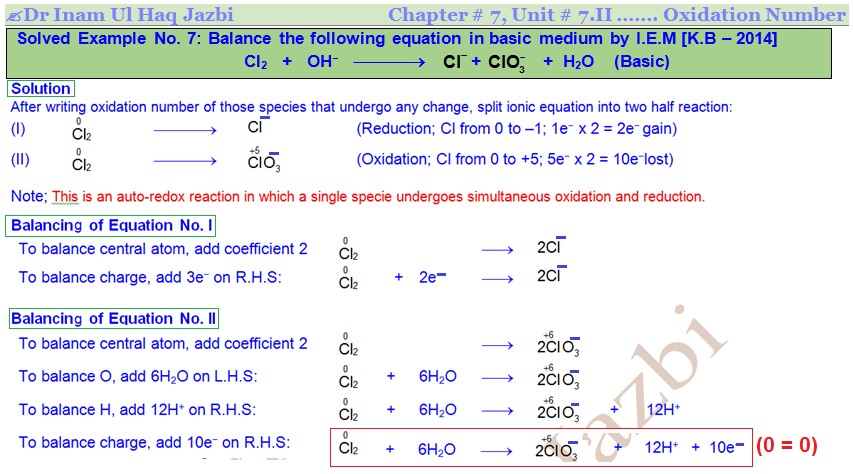
Balance the following equation in Basic medium by I.E.M.
Splitting of ionic equation into two half-reactions
After writing oxidation number of those species that undergo any change, split ionic equation into two half reactions: