Ionization
Ionization is the process of dissociation or splitting up of an electrolyte into its constituting positive and negative ions in aqueous solution or in fused state.
Introduction
A Swedish Chemist, Arrhenius in 1884 presented Theory of Electrolytic Dissociation to account for mechanism of conductivity of electrolytes, behaviour of electrolytic dissociation and phenomenon of electrolysis. [It gives not only a simple mechanism of conductivity of electrolytes but also a clear explanation of deviation of electrolytes observed in dilute solution].

The main points of this theory are summarized as follows:
AB(aq) → A⁺(aq) + B⁻(aq)
HCl(aq) → H⁺(aq) + Cl⁻(aq)
NaOH(aq) → Na⁺+(aq) + OH⁻(aq)
NaCl(aq) → Na⁺(aq) + Cl⁻(aq)
AB(aq) ⇌ A⁺(aq) + B⁻(aq)
e.g.
NaCl(aq) ⇌ Na⁺(aq) + Cl⁻(aq)
Thus ionization is a reversible process to which Law of Mass Action can be applied:
Where;
K = Ionization constant ,
[ ] = Molar conc. of ions or unionized molecules.
3. When an electric current is passed through an electrolytic solution, cations move towards the cathode and anions move towards anode. This movement of ions is called Electrolysis. [Although this term is wrong in actual meanings because ionization has already been occurred and is not due to electric current. Thus electric current does not cause ionization. The current only directs or shifts the ions towards their respective electrodes]. This movement of ions is responsible for the conduction of electricity through electrolytic solutions. These ions lose their charges on electrodes and change into neutral atoms or molecules by the gain or loss of electrons.
4. The properties of an electrolyte in solution are the properties of the ions produced in the solution. [In aqueous solution, acidic properties are due to H+ ions, basic properties are due to OH– ions and the properties of salts are the properties of ions].
5. The electrical conductivity (i.e. ability to conduct electricity which is reciprocal to resistivity) of an electrolytic solution depends upon the number of ions present in the solution which in turn is proportional to the degree of dissociation, at a particular concentration and temperature.
6. The degree of dissociation is the
extent to which the substance ionizes or the fraction of the total number of molecules present in the
ionic form.
It depends upon:
(i) Nature of electrolyte.
(ii) Nature of solvent.
(iii) Degree of dilution
(iv) Temperature.
Strong electrolytes (e.g. HCl, NaCl etc) dissociates completely in water as compared to weak electrolytes (e.g. CH3COOH, NH4OH) which ionizes to lesser extent.
The behaviour of electrolyte differs in different solvent, thus HCl is dissociated in water due to high dielectric constant (80) of water.
The more dilute the solution is, the greater will be degree of dissociation of an electrolyte as more dilute solution produces greater number of ions.
The greater the temperature, the more is the degree of dissociation.
Electrolysis
Definition of Electrolysis
Electrolysis is the process of chemical decomposition of an electrolyte in aqueous solution or molten (fused) state into its constituent ions and their deposition as neutral species at their respective electrodes by the passage of electric current with all the chemical process involved in it.
The process
of the movement of cation and anion of an electrolyte in fused state or in its
aqueous solution and their conversion into neutral species (atoms or molecules)
at their respective electrodes under the influence of an applied electric field
is called electrolysis.
OR
The process of decomposition of an electrolyte by the passage of electricity through its molten state or aqueous solution form in an electrolytic cell is called electrolysis.
Electrolysis is the process in which electrical energy is used to bring about a non-spontaneous chemical change.
Explanation
1. Electrolysis is an endothermic chemical change (decomposition) caused by the passage of an electric current through fused ionic compounds or their aqueous solutions. [Note that in the electrolytic conduction, the electrolyte is decomposed].
2. During electrolysis, metals are deposited on a cathode or hydrogen gas is released while non-metals (gases) are liberated at anode or metal making anode dissolves.
Conditions for Electrolysis
1) Electrolyte should
be in molten state or in solution form.
2) Solution should be
diluted, so degree of dissociation is greater.
3) The high
temperature favours the electrolysis.
Factors affecting products of electrolysis
The products of electrolysis mainly
depends upon
(i) nature of electrolyte (strong or weak)
(ii)Nature
of electrode (Inert or non-attacked or
reactive electrodes)
(iii) Concentration of ions
(iv) Over
potential of ions
Inert or non-attacked Electrodes
These electrodes do not react with solution of electrolyte or with the
product of
electrolysis. e.g. C (graphite), Pt
reactive electrodes
They are not used in electrolysis.
Preferential
Discharge of Ions
When there are more than one cation or anion the process of
discharge becomes competitive in nature. Discharge of any ion
requires energy and in case of several ions being present the
discharge of that ion will take place first which requires
the energy.
Uses and
Applications of Electrolysis
The process of electrolysis is used for following purposes:
1. For electroplating
of metals
2. For extraction of metals
from their ores
3. For refining of impure
metals
Uses and
Applications of Electrolysis
1) For electroplating of metals
The process of electrolysis is used for electroplating baser metals
with superior metals to make them more attractive and resistant for
corrosion. Nickel plating, chromium plating, tin plating, gold plating
etc. are various types of electroplating.
2)
For extraction of metals from their ores
3)
For refining of impure metals
Many metals are purified into pure metals by process of electrolysis.
For example, impure copper (blister copper) is purified by the process of electrolysis.
Construction and working
of an electrolytic cell
An electrolytic cell consists of electrolyte in a vessel, electrodes and a battery.
The figure shows that electrons from battery enter through cathode at which positive ions are reduced by accepting electrons. At anode negative ions loses electrons and undergoes oxidation. It means at cathode reduction occurs and oxidation takes place at anode.
Electrolytes
& its composition
The compounds which ionize into positive and negative ions in
aqueous solution conduct electricity are called electrolytes, which
always contain two types of ions namely Positively charged ion
called cation, (Na+, K+, Mg2+ etc. ) and Negatively charged ion
called anion (Cl–, SO42– etc.). These ions move randomly in their solution.
Construction
of Electrolytic Cell
Working of Electrolytic Cell or Mechanism of Electrolytic Conduction
1. When an electrolyte is fused or dissolved in water, it is ionized
and its constituents ions are free to move through the molten electrolyte or its solution.
2. When two electrodes, connected to the two poles of a battery are immersed in the electrolyte, the battery supplies electrons to one electrode (cathode).
3. The cations of the electrolyte move to cathode (negative electrode) where they receive electrons thereby changing into neutral atoms.
4. At the other electrode (anode), the battery withdraws electrons. The anions of the electrolyte rush to anode where they transfer their electrons thereby changing into neutral atoms.
5. It is this movement of ions (in opposite directions), which constitutes the electrolytic current.
6. the battery provides a source of electrical energy, pushing electrons onto the cathode and making it negatively charged . Electrons are also drawn out of the anode, making it positively charged.
Migration of Ions towards respective Electrodes
The ions of an electrolyte move toward their respective electrodes by the passage of electric current through its aqueous solution. The cations are attracted towards cathode while anions are attracted towards anode.
Discharge and Deposition of ions into Neutral Species at respective Electrodes
On reaching the electrodes, these migrating ions change into neutral species by losing or gaining electrons.
Redox Reaction
The electrolytic reaction occurring at electrodes involves reduction at cathode and oxidation at anode is called Redox Reaction or Oxidation-Reduction Reaction (ORR).
Reduction
The process that involves the gain of electrons is called Reduction.
It always takes place at cathode.
Oxidation
The process that involves the loss of electrons is called Oxidation.
It always takes place at anode.
Overall Reaction
The overall reaction is obtained by adding two half reactions i.e.
oxidation and reduction.
Predictions of Products of Electrolysis Using Inert Electrodes
(Platinum or Graphite)
Predictions of Products of Electrolysis
Using Active Electrodes
Predictions of Products of Electrolysis
Electrolytic Reactions or Cell Reactions
Electrolysis of Aqueous CuCl2
1. When cupric chloride; CuCl2 (an ionic compound) is dissolved in water, it dissociates into its ions i.e. Cu2+ and Cl –. Before application of current, these ions move randomly in water. But when the current is passed, then these ions start moving towards their respective electrodes.
2. On passing electricity through the aqueous copper chloride, electrolysis starts with the movement of cations (Cu2+) towards cathode and anions (Cl–) towards anode where they are discharged into copper metal and chlorine gas respectively.
3. When ions are changed to neutral particles, the current can no longer flow.
Cell or Electrolytic Reaction
Electrolysis of Molten Sodium Chloride
1. Solid sodium chloride does not conduct electricity (because its ions are held together tightly in regular lattice arrangement and are not free to move in the crystal) but when sodium chloride is fused at 800°C, their ions Na+ and Cl– are freed from their lattice moving freely to conduct electricity.
2. On passing electricity through the molten sodium chloride, electrolysis starts with the movement of cations (Na+) towards cathode and anions (Cl–) towards anode where they are discharged into sodium metal and chlorine gas respectively.
3. The potential required to oxidize Cl- ions to Cl2 is -1.36 volts and the potential needed to reduce Na+ ions to sodium metal is -2.71 volts. The battery used to drive this reaction must therefore have a potential of at least 4.07 volts.
Electrolytic Reaction
Definition of Electrode Potential
When a metal (electrode) is placed in aqueous solution of its own ions in a half cell, it has a tendency to lose or gain electrons and acquires either negative or positive charge with respect to solution and potential is developed between metal and solution which is named by electrode potential of metal. It is the capability or power of a metal electrode to undergo oxidation or reduction.
Nature of Electrode Potential
Electrode potential is an intrinsic or intensive property and does not depend on the size of metallic rod. Its value does not change when half-cell reaction is multiplied or divided by a co-efficient. It is independent of the amount of species in the reaction
Factors affecting
Electrode potential
1. Nature of electrode
2. Concentration of ions in solution
3. Temperature
Cell Potential
The force that causes the flow of electrons from one electrode to another electrode is called e.m.f of the cell also called cell potential.
Oxidation Potential and Reduction Potential
1. Both
oxidation and reduction potentials are equal in magnitude but opposite in sign.
2. It is not a thermodynamic property, so values of E are not additive.
Oxidation potential/ Standard Oxidation potential (SOP)
Metal atoms
of electrode have tendency to go into the solution as positive ion and leave
behind the electrons at the electrode trying make it negatively charged. The
electrode potential developed so called oxidation potential.
Oxidation potential is the potential developed at the anode by oxidation.
It is a measure of electron losing tendency of metal (to get oxidize). [In
Galvanic cell, species or substances having smaller reduction potential is
oxidized and act as anode]. Thus, oxidation potential of the half-cell
undergoing oxidation (M ⇌ M+ + 1e–)
is represented by:
For example;
in Zn-Cu Galvanic cell, Zn acts as anode while Cu acts as cathode. Thus Zn
oxidizes into Zn2+ ions by losing electrons going into the solution
surrounding the anode (i.e. ZnSO4). The oxidation potential of Zn
anode is +0.76 V. However, in Mg-Zn Galvanic cell where Zn acts as cathode, its
reduction potential is –
0.76 V.
Reduction Potential/Standard Reduction Potential (SRP)
Metal ions
present in the solution have tendency to deposit on the electrode by accepting
electrons from it making the electrode positively charged. The electrode
potential developed so called reduction potential.
Reduction
Potential is the potential developed at the cathode by reduction. It is a
measure of the tendency of metal for reduction to occur. [In Galvanic cell,
species or substances having greater reduction potential is reduced and act as cathode].Thus,
reduction potential of the half-cell undergoing reduction, (M+ +1e–
⇌ M) is
represented by:
For example; in Zn-Cu Galvanic cell, Zn acts as anode while Cu acts as cathode. Thus Cu2+ ions reduce at cathode by gaining electrons from the cathode and convert into Cu atoms which deposit on the cathode. The reduction potential of Cu cathode is +0.34 V. However, in Cu-Ag Galvanic cell where Cu acts as anode, its oxidation potential is – 0.34 V.
According to IUPAC convention, the reduction potential alone be called as the electrode potential unless it is specifically mentioned.
The
oxidation potential and the reduction potential of an electrode have the same
value but differ in signs [Oxidation potential are obtained by reversing the
sign of reduction potential].
Electromotive Force (e.m.f)
It is the difference between the
electrode potentials of two half-cells and cause flow of current from electrode
at higher potential to electrode at lower potential. It is also the measure of
free energy change. Standard emf of a cell is given by,
Standard Electrode Potential (SEP)
The potential of an electrode (half-cell) determined by comparing it with reference electrode called Standard Hydrogen Electrode (S.H.E.) at standard conditions (at 298 K, 1 atm. pressure and 1M concentration) is called Standard Electrode Potential denoted by Eo. It is the potential difference developed between metal electrode and solution of ions of unit molarity (1M) at 1 atm pressure and 25°C (298 K) is called standard electrode potential.
It is a measure of tendency of a metallic electrode to lose or gain electrons when it is in contact with a solution of its own ions of 1 M concentration at 25oC and 1 atm. pressure.
Since absolute electrode potential cannot be measured, so the relative electrode potential is determined. The potential of a single electrode can be measured by coupling (comparing) it with a standard half-cell whose electrode (reduction) potential has been assigned a constant value. For this purpose a reference electrode called Standard Hydrogen Electrode (SHE) is used whose potential is arbitrarily chosen as 0.000 volt.
When an electrode is combined with S.H.E., it either undergoes oxidation or reduction. At the electrodes having greater reduction potential than 0.000, reduction takes place and SHE undergoes oxidation and acts as anode. [e.g. SHE acts as anode when it is combined with copper electrode. In this case, oxidation will take place at SHE]. At the electrodes having smaller reduction potential than 0.000, oxidation takes place and SHE undergoes reduction and acts as cathode [e.g. SHE acts as cathode when it is combined with zinc electrode. In this case reduction takes place at SHE].
Reference Electrode
The electrode of known potential is called reference electrode. It may be primary reference electrode like hydrogen electrode or secondary reference electrode like calomel electrode.
Primary Reference
Electrodes
The electrode potential is found out by coupling the electrode with a primary reference electrode, the potential of which is arbitrarily fixed as zero. The important primary reference electrode used is a standard hydrogen electrode or normal hydrogen electrode.
Secondary Reference
electrode
The use of SHE is difficult, because it is difficult to maintain 1M H+ ion concentration and the pressure of the gas at one atmosphere. Also the electrode will easily get poisoned in case of traces of impurities in the gas and hence other reference electrode called secondary reference electrodes are used. e.g. Saturated calomel electrode (saturated KCl)
Standard Hydrogen Electrode (SHE)/Normal Hydrogen Electrode (NHE)
In order to determine the electrode potential of an unknown electrode, some standard electrodes are used like
(i) Standard Electrode
(ii) Calomel electrode
It is impossible to measure the absolute electrode potential of a single electrode, so the relative electrode potential is determined by connecting an electrode (whose electrode potential is to be measured) with a standard half-cell whose electrode (reduction) potential has been assigned a constant value. For this purpose a reference electrode called Standard Hydrogen Half-Cell or Standard Hydrogen Electrode (SHE) is selected whose potential is arbitrary chosen as 0.000 volt for coupling with unknown half-cell.
Standard Hydrogen Electrode (SHE) is used as reference electrode to
determine the reduction potential of different electrodes. To define an
electrochemical ‘Sea-Level’, chemists have chosen a reference standard half-cell
called the standard hydrogen electrode (SHE).
An SHE consists of a platinum plate (inert electrode) coated
with a layer of finely divided platinum black electrolytically (to give it a
large surface area) immersed in one molar (1 M) HCl/H2SO4
(H+ ions) solution encased in a glass hood (tube) so that H2
gas at standard state conditions (i.e. 1 atm pressure and 25°C/ 298 K
temperature) can bubble over the platinum electrode. The platinum
acts as an electrical conductor and also facilitates the attainment of
equilibrium between the gas and its ion in solution. [The platinum adsorbs H2
gas on its surface and the platinum coated with hydrogen behaves as if it was
made entirely of hydrogen. An equilibrium is established between adsorbed layer
of hydrogen and H+ ion in the solution].
Standard hydrogen electrode (SHE) Standard hydrogen electrode (SHE) also known as normal hydrogen electrode (NHE), consists of platinum wire, carrying platinum foil coated with finely divided platinum black. The wire is sealed into a glass tube placed in beaker containing 1 M HCl. The hydrogen gas at 1 atm pressure is bubbled through the solution at 298K. Half-cell is pt H2 (1 atm) H+ (1 M)
The standard electrode potential (Eoxidation or Ereduction) of the SHE is arbitrary taken as zero volt at all temperature. The possible SHE half reactions are:
SHE can acts
as cathode as well as anode depends on the other electrode.
function of platinised platinum
1. It provides support surface for establishing an equilibrium between H2 gas and H+ ions as soon as possible.
H2 (g) ⇌ 2H+(aq) + 2e–
2. It allows H2 gas to be adsorbed on its surface (the platinum coated with hydrogen behaves as if it were made entirely of hydrogen).
3. It acts as inert metal connection to H+/H2 system but it does not take part in the chemical reaction (i.e. it has no tendency to form ions itself).
4. It also provides the necessary electrical connection to carry away or supply electrons.
Drawbacks
Its main drawbacks are
1. It is difficult to maintain 1 atm
pressure of H2 gas.
2. It is difficult to maintain H+
ion concentration 1 M.
3. The platinum electrode is easily poisoned by traces of impurities.
Hence, calomel electrodes are conveniently used as reference electrodes, It consists of mercury in contact with Hg2 Cl2 (calomel) paste in a solution of KCl.
Measurement of Electrode Potential of Zinc - SHE Cell
Construction of Galvanic Cell
The
potential of a single electrode (like Zn) can be measured by connecting it with
SHE. For this purpose, a Galvanic cell consisting of two half-cells (each
containing an electrode dipped in solution of its own ions) is constructed.
1). Anode Half Cell; zinc metal dipped in 1M ZnSO4 solution.
2). Cathode Half Cell, SHE (inert Pt electrode immersed in
1M HCl solution in a current of H2 gas at 1 atm)
3). Salt bridge; inert electrolyte (KCl jelly) completes the circuit and prevents
mixing of solutions
4). voltmeter; Both electrodes are connected with voltmeter giving positive cell potential (E°).
1). Anode
Half Cell
On the left is placed a half cell consisting of zinc metal (whose electrode potential is to be measured) dipped in 1M ZnSO4 solution.
2). Cathode
Half Cell
On the right, is arranged SHE consisting of an inert platinum electrode immersed in 1M HCl solution through which H2 gas is bubbled at 1 atm. pressure.
3). Salt bridge
The two half cells are connected by salt bridge made of KCl jelly (inert electrolyte) completes the circuit between two half cells and prevents mixing of solutions.
4). voltmeter
Both
electrodes are connected with the terminals of a voltmeter in such a way that
measured cell potential (E°) cell be positive. [The electrode connected with
–ive terminal of voltmeter would be negative electrode i.e. anode whereas the
electrode connected with positive terminal of voltmeter would be positive
electrode i.e. cathode].
Principle of Working
1). Anode
Reaction
As the cell operates, the mass of zinc electrode decreases and concentration of Zn2+ ions increase around the zinc electrode.
2). Cathode
Reaction
The H+ ions
concentration decreases in SHE half-cell and H2 is produced.
3). Function
of Salt bridge
Due to movement of ions (i.e. Zn2+ and H+ ions), charge imbalance creates in half cells which ceases electrode reactions thereby halting electron flow through the wire. Thus ions of inert electrolyte from the salt bridge migrate to neutralize the charge in the cell compartments. Anions move towards the anode and cations move towards the cathode.
4). Cell
Potential
The voltage or cell potential or E° cell given by voltmeter is found to be +0.76 volt, when zinc electrode is connected with the negative terminal of voltmeter and SHE with positive terminal of voltmeter. [This shows that zinc electrode acts as anode i.e. oxidation takes place at zinc electrode while SHE acts as cathode i.e. at SHE reduction takes place. Thus zinc electrode is negative with respect to SHE].
Cell Reactions
As the reduction potential is the reverse of oxidation potential, therefore, reduction potential of zinc would be –0.76 volt. The negative sign signifies that actually the reaction at zinc electrode occurs in opposite direction i.e. it is the oxidation rather than reduction which occurs at zinc.
Cell Diagram
In this cell zinc reduces H+ ion to H2 gas and itself oxidizes to Zn2+ ions. Thus flow of electrons takes place from zinc to SHE.
Summary
of Zinc-SHE cell
SHE may act
as a cathode or anode depends on other electrode. If Zn half-cell is connected
with SHE, Zn will oxidize into Zn2+ ions thereby acting as anode while
SHE get reduced into H2 gas acting as cathode. The reading in the
voltmeter (+0.76 V) will be oxidation potential of the other electrode (i.e.
Zn) as the electrode (reduction) potential of SHE is 0.000 V (The reduction
potential value is same as oxidation potential but with opposite sign i.e.
-0.76 V. The – sign implies that the reaction at Zn electrode is oxidation
rather than reduction). The reactions occur are:
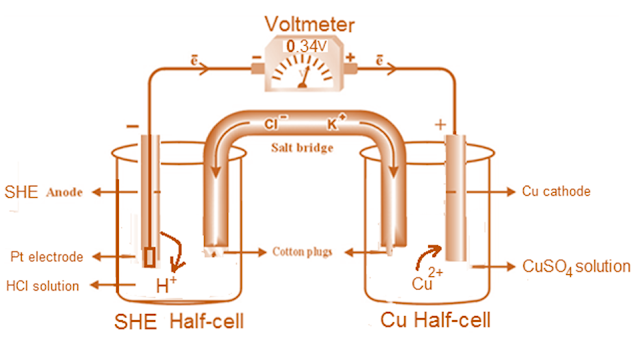
Measurement of Electrode Potential of Copper (SHE-Copper Cell)
Construction of Galvanic Cell
The potential of a single
electrode (like Cu) can be measured by connecting it with SHE. For this
purpose, a Galvanic cell consisting of two half-cells (each containing an
electrode dipped in solution of its own ions) is constructed.
1). Anode Half Cell; SHE (inert Pt electrode immersed in 1M HCl
solution in a current of H2 gas at 1 atm)
2). Cathode Half Cell, Copper
metal dipped in 1M CuSO4 solution.
3). Salt bridge; inert
electrolyte (KCl jelly) completes the circuit and prevents mixing of solutions
4). voltmeter; Both electrodes are connected with voltmeter giving positive cell potential (E°).
1). Anode
Half Cell
On the left, is arranged SHE consisting of an inert platinum electrode immersed in 1M HCl solution through which H2 gas is bubbled at 1 atm. pressure.
2). Cathode
Half Cell
On the right is placed a half cell consisting of copper metal (whose electrode potential is to be measured) dipped in 1M CuSO4 solution.
3). Salt bridge
The two half cells are connected by salt bridge made of KCl jelly (inert electrolyte) completes the circuit between two half cells and prevents mixing of solutions.
4). voltmeter
Both electrodes are connected with the terminals of a voltmeter in such a way that measured cell potential (E°) cell be positive. [The electrode connected with –ive terminal of voltmeter would be negative electrode i.e. anode whereas the electrode connected with positive terminal of voltmeter would be positive electrode i.e. cathode].
Principle of Working
1). Anode
Reaction
As the cell operates, the H+ ions concentration in SHE half-cell increases and H2 gas is consumed.
2). Cathode
Reaction
The concentration of Cu2+ ions increase around the copper electrode.
3). Function
of Salt bridge
Due to movement of ions (i.e. Cu2+ and H+ ions), charge imbalance creates in half-cells which ceases electrode reactions thereby halting electron flow through the wire. Thus ions of inert electrolyte from the salt bridge migrate to neutralize the charge in the cell compartments. Anions move towards the anode and cations move towards the cathode.
4). Cell
Potential
The voltage or cell potential or E° cell given by voltmeter is found to be +0.34 volt, when SHE is connected with the negative terminal of voltmeter and copper electrode with positive terminal of voltmeter. [This shows that SHE acts as anode i.e. oxidation takes place at SHE while copper electrode acts as cathode i.e. at copper electrode reduction takes place. Thus copper electrode is positive with respect to SHE].
Cell Reactions
As the oxidation potential is the reverse of reduction potential, therefore, oxidation potential of copper would be –0.34 volt. The negative sign signifies that actually the reaction at copper electrode occurs in opposite direction i.e. it is the reduction rather than oxidation which occurs at copper.
Cell Diagram
In this cell,
SHE (H2) reduces Cu2+ ion to copper metal and itself oxidizes
to H+ ions. Thus flow of
electrons takes place from SHE to copper.
Summary
of SHE-Cu cell
SHE may act as a cathode or anode depends on other electrode. If Cu half-cell is connected with SHE, Cu2+ ions are reduced into Cu thereby Cu acting as cathode while SHE gets oxidized into H+ ion acting as anode. The reading in the voltmeter (+0.34 V) will be the reduction potential of the other electrode (i.e. Cu) as the electrode (oxidation) potential of SHE is 0.000 V (The oxidation potential value is same as reduction potential but with opposite sign i.e. – 0.34 V. The – sign implies that the reaction at Cu electrode is reduction rather than oxidation). The reactions occur are:
Electrochemical Series (E.C.S)
Definition of E.C.S
Consequently,
the strongest reducing agents are located in the upper right of the table (Li,
Cs, K, Ba, Sr, Ca, Na, Mg, Be, Al, Zn etc.) and the strongest oxidizing agents
are found in the lower left of the table (F2, O3, MnO4‒,
Cr2O72‒ etc.). All metals above hydrogen have
negative reduction potentials.
Features
of E.C.S
Different elements have
different tendencies to lose or gain electrons i.e. to be oxidized or reduced.
For determination of standard reduction potential of elements, electrode
potential of SHE is used as a reference point.
1. In the series, all the elements above
SHE have negative reduction
potential and positive oxidation potential.
2. In the series, all the elements below
SHE have positive reduction
potential and negative oxidation potential.
3. Oxidation potential of electrode is numerically equal to reduction potential but with opposite sign.
4. Metals placed above hydrogen in E.C.S. have lower (negative) reduction potential acts as anode (i.e. they are oxidized) while metals (or non-metals) placed below hydrogen in E.C.S. have greater (positive) reduction potential acts as cathode (i.e. they are reduced).
5. From top to bottom in E.C.S, the reducing strength decreases and oxidizing strength increases. Thus Li is the strongest reducing agent and F2 is the strongest oxidizing agent.
6. Position of metals in E.C.S shows the
order of their reactivity. Metal displaces metal lying below them in the series from its solution of
their salts.
Applications of E.C.S
E.C.S is used: