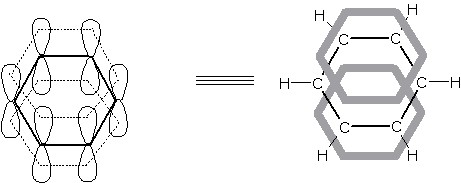
Molecular Orbital Treatment of Benzene
Total Valence Electrons in Benzene
There are six carbon and six hydrogen atoms in benzene. Each carbon atom contributes four and each hydrogen atom contributes one valence electrons in benzene, so there are thirty valence electrons in benzene (C = 24, H = 6).
Nature of Hybridization
According to molecular orbital treatment, the nature of hybridization in each of the six carbon atoms of benzene should be sp2 as each carbon atom is linked with three other atoms (two carbons and one hydrogen).
Evidences of sp2-Hybridization
Formation of sp2-Hybrid Orbital
The hybridization in benzene can be explained by considering the electronic configuration of carbon. sp2-orbital hybridization in each carbon atom forms three sp2-hybrid orbitals at the mutual angle of 120°. The unhybridized 2pz orbital of each carbon remain at right angle to the plane of sp2-orbitals. Thus each carbon has three hybrid and one unhybrid orbitals.
Sigma Bond Formation in Benzene
Two of three sp2-hybrid orbitals of each carbon atom overlap linearly with sp2-hybrid orbitals of two adjacent carbon atoms (sp2-sp2 overlap) forming two C–C sigma bonds while remaining one sp2-orbital of each carbon atom overlap linearly with 1s orbital of a hydrogen atom forming one C–H sigma bond. Thus, there are six C–C s-bonds (as there are six carbons) and six C–H s-bonds in benzene i.e. there are total twelve sigma bonds. [Twelve electrons are thus located in six C–C s-bonds and another twelve to six C–H s-bonds. This account for twenty four electrons. The remaining six electrons are present in the form of p-electrons in unhybridized orbital].
Bond Length Data
The Kekule structure of benzene as cyclohexa-1,3,5-triene suggests two bonds lengths for the separate single and double bonds.
C – C bond length = 154 pm
C = C bond length = 134 pm
Experiment shows only one C – C bond length of 139 pm, between the bond the bond lengths for single and double carbon-carbon bonds.
This shows that each carbon=carbon bond in the benzene rings is intermediate between a single and a double bond.
Pi Bond Formation in Benzene
[Each carbon atom of benzene has one unhybridized 2pz1 orbital. Thus six carbon atoms have six unhybridized orbitals which are parallel to each other and are perpendicular to the plane of sigma-electrons; having one lobe above and other below the plane]. The unhybridized pz-orbitals of each carbon undergo side-wise or lateral overlapping with one another (as in alkene) to give rise to three pi (p) bonds.
Different Ways of Overlapping Unhybrid Pz-Orbitals
The 2pz1 electron of two adjacent carbon atoms are shared forming a p-molecular orbital. The electrons in these p-orbitals can be shared in two ways as shown in the figure:-
Formation of Delocalized p-Bond
In actual practice, the electrons of unhybridized orbitals make parallel overlap above and below the benzene ring forming six p-bonds; enveloping the six C–C s bonds. By virtue of above treatment, the six p-electrons get associated with all the carbon atoms forming a continuous circular sheath of p-electronic cloud (delocalized/diffused electronic cloud) above and below the plane of the ring.
The overlap of these p-orbitals result in the formation of a fully-delocalized p-bond which extends all over the six carbon atoms. [These p-electrons are not confined or restricted to a particular carbon atom and changing their position continuously]. These electrons of p-orbitals are also known as Diffused or Delocalized electrons while the process of formation of p-bond spreading all over six carbon atoms is known as Delocalization. Due to this delocalization of p-electrons in benzene, a more stronger p-bond than normal p bond is obtained and more stable molecule is formed.
Conclusion
The molecular orbital approach clearly shows that the continuous sheath of delocalized p-electrons is responsible for extra-stability and peculiar behaviour of benzene molecule. The physical evidences are in complete agreement with M.O.T.