Important Objectives for Chemistry Paper XII 2023
Inorganic Section
1. Hydrated copper
sulphate is a blue crystalline solid.
2. The formula of baking soda is NaHCO3.
3. The outer electronic configuration
of copper is 4s1 3d10.
4. The chemical name of lunar caustic
is silver nitrate (AgNO3).
5. Sub-Group B elements are called transition elements.
6. The oxide of Aluminium is amphoteric.
7. The oxide of Zinc is amphoteric.
8. Elements which follow Lanthanum are
called Lanthanides.
9. Elements which follow Actinium are
called Actinides.
10. Ionic hydrides are also called true/salt like/saline hydrides.
11. The formula of Hypo is Na2S2O3.5H2O (Sodium
Thiosulphate).
12. The formula of Borax is Na2B4O7.10H2O Sodium
Tetraborate).
13. When steam is passed over red hot
iron, Magnetic Iron Oxide (Fe3O4) is formed along with H2 gas.
14. NaOH absorbs CO gas to form Sodium Formate (HCOONa).
15. KOH absorbs CO2 gas to form
Potassium Carbonate (K2CO3).
16. The formula of Gypsum is CaSO4.2H2O.
17. The formula of Epsom Salt is MgSO4.7H2O.
18. The formula of Plaster of Paris is CaSO4.½H2O.
19. The formula of Cryolite is Na3AlF6.
20. The formula of Ortho Boric Acid H3BO3.
21. The formula of Colemanite is Ca2B6O11.5H2O.
22. CrO2Cl2 is the
formula of Chromyl Chloride.
23. K2[HgI4] is the
formula of Nesseler’s Reagent.
24. Deutrium is also called Heavy Hydrogen.
25. Tritium is the radioactive isotope
of hydrogen.
26. s-block elements behave as powerful
reducing agents because of their high reduction potential.
27. CaOCl2 is the formula of
Bleaching Powder.
28. in
acidic medium, KMnO4 oxidizes KI to iodine (I2).
29. CuSO4 forms brown ppt. of
copper ferrocyanide with potassium ferrocyanide.
30. E.D.T.A. is an example of Hexadentate ligand.
31. Ligands are generally called Lewis Bases.
32. Zn is diamagnetic as all the electrons are
paired.
33. The outer electronic configuration of
Cr is 4s1
3d5.
34. High B.P. and viscosity of H2SO4
are due to presence of Hydrogen Bonding.
35. 90% concentrated H2SO4
oxidizes Zn to ZnSO4 while itself reduces to H2S.
36. H2S2O7
is the formula of Oleum or Pyrosulphuric Acid.
37. The formula of Stibnite is Sb2S3.
38. The compounds of zinc are white.
39. The formula of chrome red pigment is Pb2CrO5 or PbCrO4.PbO.
40. The formula of chrome yellow pigment
is PbCrO4.
41. The formula of red lead pigment
(Sandhur) is Pb3O4
or 2PbO.PbO2.
42. The formula of white lead pigment is 2PbCO3.Pb(OH)2.
43. In diamond, each carbon is sp3-hybridized.
44. In graphite, each carbon is sp2-hybridized.
45. Graphite is a good conductor of heat and electricity.
46. Diamond is non-conductor of electricity.
47. Duralumin contains 95% Al, 4% Cu, 0.5% Mg
and 0.5% Ni.
48. Bauxite ore has the formula Al2O3.nH2O.
49. At 160°C boric acid is changed into Pyroboric Acid.
50. The formula of washing soda is Na2CO3.10H2O.
51. Ordinary hydrogen
is unique in not having neutron.
52.* Energy of t2g orbital is less than that of eg orbital.
53. The refractive index of diamond is 2.35.
54. Graphite conducts electricity due to delocalized electrons.
55. The angle between
H-S-H in H2S molecule is 92.2°.
56. The mixture of
aluminium powder and aluminium nitrate is known as ammonal.
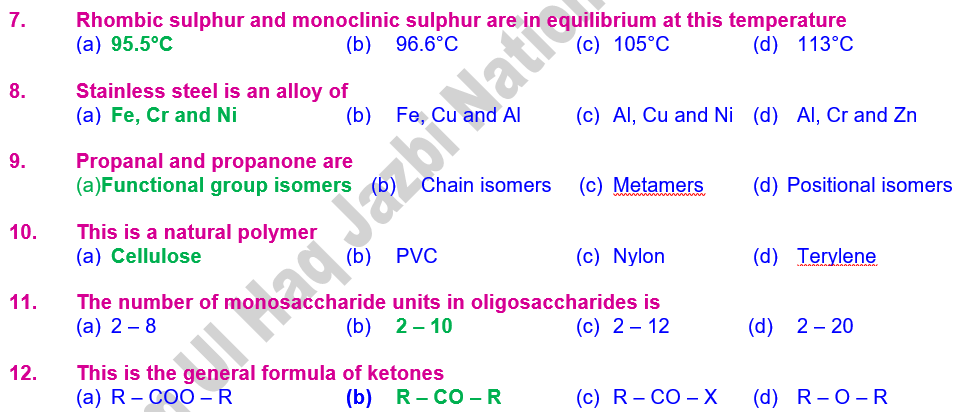
57. The transition
temperature of rhombic sulphur and monoclinic sulphur is 95.5°C.
58. The structure of
hydrogen sulphide is tetrahedral or angular or distorted
tetrahedral.
59. Zinc hydroxide is
soluble in excess of NaOH due to formation of soluble tetrahdyroxozincate(II)ion.
60. Mendeleev states
that the properties of elements are periodic function of their atomic
masses.
61. The electron
population ratio of aluminum is less than that of boron.
62. The I.P. of nitrogen is more than that of oxygen due to
presence of stable half-filled orbitals.
63. Ferric ion is more stable than ferrous ion
due to presence of stable half-filled orbitals.
64. The temperature at which a-sulphur and b-sulphur exist in equilibrium is called transition temperature that is 96.5°C.
65. The outer shell
electronic configuration of group VIIA and VIIB are ns2 np5 and ns2 (n-1)d5 respectively.
66. Octet rule is
fulfilled by all noble gases except He.
67. Hydrides of VI
and VII group are acidic.
68. Hydrides of V
group are basic.
69. Hydrides of III
and IV group are neutral.
70. The nucleus of tritium consists of one proton and
two neutrons
71. The metal forming
superoxides are K, Rb and Cs.
72. Antidote of H2S
poisoning is very dilute chlorine.
73. Galvanized iron
means iron coated with zinc.
74. The product of
heating boric acid to 140°C is Pyroboric
acid.
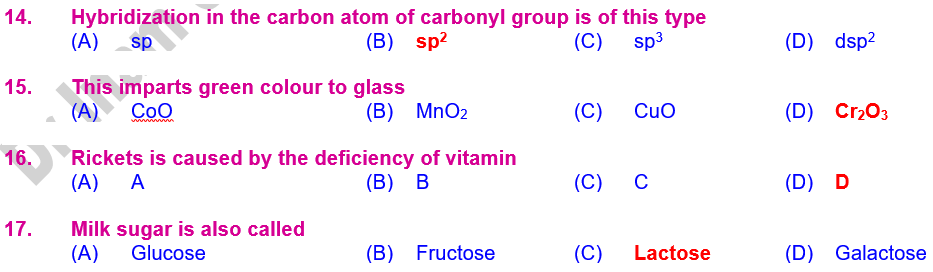
75. Cr2O3 imparts green colour to glass.
76. The element
belonging to group VA and 3rd period has the atomic number 15.
77. The element
belonging to group VA and 4th period has the atomic number 33.
78. The element
belonging to group VIA and 3rd period has the atomic number 16.
79. The element
belonging to group VIA and 4th period has the atomic number 34.
80. the number of neutrons in protium is zero.
81. the number of neutrons in deuterium is 1.
82. the number of neutrons in tritium is 2.
83. Both crystalline
forms of sulphur exist at this transition temperature 95.5°.
84. Ammonal is the mixture of
aluminium nitrate and aluminum powder.
85. Elements of group
IB are called Coinage
metals.
86. Elements of group
IA are called alkali
metals.
87. Metallic hydrides are also known as
Interstitial hydrides or non-stoichiometric hydrides.
88.. Chemical
composition of colemanite is Ca2B6O11.5H2O
89. Coinage metals
are elements of IB group and these include Cu, Ag, Au
90. Having
half-filled valence shell, hydrogen resembles the elements of IVA group
91. Plaster of Paris
is obtained by heating Gypsum
92. Sodium burns with
excess of oxygen to form its Peroxide
93. Royal water is a
mixture in the ratio of 1:3 by volume of HNO3
and HCl
94. Transition temperature is the temperature at which
two allotropic forms of an element exist in equilibrium state
95. Ruby is an ore of Aluminium
96. H2S is
a Reducing agent
97. Sodium amalgam is
an alloy of Sodium and mercury
98. Stainless steel
is an alloy of Fe, Cr and Ni
99. Kipp’s apparatus
is used to prepare H2S
100. The formula of
dolomite is MgCO3.CaCO3
101. EDTA is Hexadentate type of ligand
102. The ratio of
electrons, protons and neutrons in deuterium is 1:1:1
103. The ratio of
electrons, protons and neutrons in tritium is 1:1:2
104. The ratio of
electrons, protons and neutrons in protium is 1:1:0
105. Aluminium bronze
contains 10% Al and 90% Cu
106. N2 gas
liquefies at a temperature -196°C
107. Hypo is used as a Fixer.
Organic Section
1. A dipolar charged but on overall electrically neutral ion is called zwitterions.
2. Animal starch is known as glycogen.
3. Vitamin B2 is known as riboflavin.
4 Resorcinol is not an alcohol but
it is a/an phenol.
5. The carbohydrates containing
aldehydic group are called aldoses.
6. There are four isomeric forms of butyl
radical.
7. Glucose and fructose are functional
group
isomers.
8. Bakelite is an example of thermosetting
plastic.
9. Monosubstituted benzenes exist in one isomeric form.
10. Xylenes have three positional isomers.
11. Bakelite is a polymer of phenol and
formaldehyde.
12. Polyethene is a polymer of ethene.
13. Nylon is a polymer of Adipic
Acid (Hexane-1,6-dioic acid) and hexamethylene diamine.
14. Water soluble vitamins are B-complex and vitamin-C.
15. Fat soluble vitamins are A,
D, E
and K.
16. Cannizaro’s reaction is given by only formaldehyde and benzaldehyde.
17. Detergents are soapless cleansing
agent.
18. The deficiency of Vitamin D causes Rickets.
19. Vitamin B1 or Thiamine
deficiency causes Beri Beri.
20. Vitamin B2 or Riboflavin
deficiency causes skin disorders.
21. Niacin or Nicotnic Acid deficiency
causes Diarrhorea and gastro-intestinal
disorders.
22. Vitamin C or Ascorbic Acid lowers the body resistance to
infection.
23. The reduction of
ethyl bromide with metallic sodium yield normalbutane.
24. Sucrose is a non-reducing sugar.
25. tertiary alkyl halide reacts by SN1
mechanism and primary alkyl halides by SN2
mechanism.
26. The reaction between metallic sodium
and alkyl halides is called Wurtz
Reaction.
27. The fractional distillation of crude
petroleum yield only 20% petrol.
28. The deficiency of Insulin causes diabetes.
29. Renin is active in the stomach of
young children.
30. Compounds with general formula CnH2n+2O
are called Alcohols
and Ethers.
31. Compounds with general formula CnH2nO2
are called Carboxylic
Acids and Esters.
32. Compounds with general formula CnH2nO
are called Aldehydes
and Ketones and oxiranes.
33. CnH2n-2 is the general formula of
Alkyne and cylcoalkenes.
34. CnH2n is the general formula of
Alkene and cylcoalkanes.
35. CnH2n+2 is the general formula of
Alkane.
36. The hybridization in Alkane is sp3.
37. The hybridization in Alkene is sp2.
38. The hybridization in Alkyne is sp.
39. The hybridization in Benzene is sp2.
40. The hybridization in Alcohol is sp3.
41. Acetylene is acidic in nature due to terminal
active hydrogen.
42. Hot KMnO4 oxidizes
acetylene to oxalic
acid.
43. Cold KMnO4 oxidizes
acetylene to formic
acid.
44. KMnO4 oxidizes ethene to Ethylene glycol.
45. Halogenation of alkane is an example
of photochemical (chain)
reaction.
46. Destructive distillation of coal tar
is the main source of Aromatic compounds.
47. Alkanes are also called Paraffins.
48. Alkenes are also called Olefins.
49. CnH2n+1 is the
general formula of Alkyl
Group.
50. Alicyclic compounds have general
formula CnH2n behave like aliphatic compounds.
51. Glass is regarded as super-cooled liquid.
52. The formula of metaformaldehyde is (CH2O)3.
53. The formula of
paraldehyde is (CH3CHO)3.
54. Only methyl ketones undergo haloform reaction.
55.Haloform reaction
is given by any carbonyl compounds containing acetyl group (-COCH3).
56. Formaldehyde and
benzaldehyde do not undergo haloform reaction due to lack of acetyl group.
58.Aldehydes are
generally prepared by the controlled oxidation or dehydrogenation of primary alcohols.
59. The alcoholic
concentration of fermented material cannot exceed than 15%.
60. Phenol is acidic in nature.
61. Esterification is an acid-catalyzed reversible condensation of an acid and an
alcohol into alkyl alkanoate.
62. Destructive distillation
(Carbonization) of coal yields coal gas (mixture of CH4,
H2 and other gases), coal tar coke and ammonical liquors.
63. Main function of vitamin A is to form visual pigment and its deficiency causes night blindness/ xerophthealmia.
64. Vitamin-K (Antihaemorrhagic compound)
helps the synthesis of Prothrombin & other blood clotting factor in liver.
65. Glucose, Fructose, Galactose
(Monosaccharides), Lactose, Maltose (D.S.) are the examples of Reducing Sugars.
66. Methanal
trimerises on heating in traces of H2SO4 to give cyclic
addition trimer called metaformaldehyde.
67. Terylene is a condensation polymer
68. PVC, PVA, polythene etc. are addition polymers.
69. The number of
isomers of pentane are 3.
70. The number of
isomers of hexane are 5.
71. The number of
isomers of heptane are 9.
72. Propanone and
propanal are Functional isomers.
73. Ethanol and
methoxymethane are Functional isomers.
74. Acetic acid and
methyl formate are Functional isomers.
75. BF3 is
an electrophile.
76. reduction of aldehyde gives primary alcohol.
77. reduction of ketone gives secondary alcohol.
78. Hybridization in
the carbon atom of carbonyl group is of sp2 type
79. Rickets is caused
by the deficiency of vitamin D.
80. Milk sugar is
also called Lactose.
81. Fruit sugar is
also called fructose.
82. The functional
group is RSH is Thioalcohol.
83. The functional
group is RSN is
nitrile.
84. Cycloalkanes have
the general formula CnH2n
85. Exoxides have the
general formula
CnH2nO
86. Another name of
methane is Marsh gas.
87. The most stable
carbonium ion is R3C+ (tertiary carbonium ion).
88. Rectified spirit
contains alcohol 92-95%
89. Formalin is used as a preservative for biological specimens.
90. Glycogen is a Polysaccharide.
91. Starch is a Polysaccharide.
92. Citrus fruits are
important source of vitamin C
93. The first step is
similar in SN1 and E1 mechanisms.
94. In acetone, the
number of bonds are Nine σ and one π
95. Octane number is
related to Gasoline
96. The reagent
converts acetic acid into acetyl chloride is SOCl2
97. Retinol is not a
member of vitamin B-complex
98. The commercial
name of phenol-formaldehyde polymer is Bakelite
99. Cellulose is
a natural polymer
100. The number of
monosaccharide units in oligosaccharides is 2 – 10
101. Another name for
wood spirit is Methyl alcohol
102. Cod liver oil is a
source of Vitamin A
103. HF is
used for etching of glass
104. Ethyl acetate is present in Pineapple
105. (C2H5)4Pb is used to increase the
octane number and efficiency of petrol.
Karachi Board MCQs 2021
Q1. Choose the best correct answer for each
from the given options
Karachi Board MCQs 2020 (No paper due to Covid)
Karachi Board MCQs 2019
Choose the best correct answer for each
from the given options:
Karachi Board MCQs 2018
Choose the best correct answer for each from the given options:
Karachi Board MCQs 2017
Q1. Choose the best correct answer for each
from the given options:
Karachi Board MCQs 2016
Choose the best correct answer for each
from the given options:
Karachi Board MCQs 2015
Choose the best correct answer for each
from the given options: