General Methods of Preparation of Carboxylic acids
1. By complete oxidation of primary alcohols by acidified K2Cr2O7
2. By oxidation of aldehydes by acidified K2Cr2O7
3. By oxidation of Ketones by acidified K2Cr2O7
4. By the carbonation of Grignard’s reagent with solid carbon dioxide followed by acidification
5. By the acid-catalyzed hydrolysis of esters with water in acidic medium
6. By the acid-catalyzed or base-catalyzed hydrolysis of alkyl nitrile (obtained from RX or RMgX)
7. By the oxidative Cleavage of alkenes

primary alcohol on complete oxidation by acidified K2Cr2O7 first forming corresponding aldehyde and finally corresponding carboxylic acid.
Aldehydes are easily oxidized to corresponding carboxylic acids by acidified potassium dichromate. [Aldehydes are more readily oxidized than ketones due to the presence of active H-atom attached to carbonyl carbon.]
Under strong oxidizing conditions, ketones are oxidized carboxylic acid having one carbon atom less then parent ketone.
Esters hydrolyze with water in acidic medium forming carboxylic acid and alcohol.
Grignard’s reagent or alkyl magnesium halide (RMgX) reacts with solid CO2 as a nucleophilic addition reaction to the electrophilic carbon of CO2 to give an intermediate addition product (halo magnesium salt of acid) which on acidification with aqueous HX forming next higher carboxylic acid [which contains one carbon more than Grignard’s reagent]. This reaction is known as Carbonation or carboxylation of Grignard’s reagent. [This method is especially used for making carboxylic acid form tertiary alcohol].
Specific Methods for the preparation of Acetic acid
1. By Destructive Distillation of Wood (From pyroligneous acid)
Acetic acid is obtained by heating wood in the absence of air until it has thermally decomposed. The strongly acidic tarry distillate Pyroligneous Acid (Wood vinegar) is obtained which contains methanol, acetone, acetic acid, methyl acetate, etc. Acetic acid is separated by the fractional distillation of this liquid at 118°C.
2. Formation of acetic acid by hydration of ethyne
Acetic acid is produced by the oxidation of acetaldehyde obtained by passing acetylene over dilute H2SO4 and HgSO4 at 75°C [to produce an unstable intermediate, hydroxy olefin (ethenol) that on rearrangement forms ethanal that oxidizes by acidified potassium dichromate to acetic acid].
Chemical Properties of Carboxylic acids
Carboxylic acid undergo following chemical reactions:
1. As a weak monobasic acid
2. Esterification
3. Decarboxylation
4. Reaction with phosphorus halides and thionyl chloride
The carboxyl group shows the chemistry of both carbonyl group and hydroxyl group. In most reactions, the carboxyl group is retained. However, the reactivity of these is due to the presence of carbonyl group
As R group increases, the reactivity decreases. Formic acid is more reactive due to absence of R group.
Reactivity of Carboxyl Group
1. Carbonyl group is electron-withdrawing; it increases the polarity of – OH group.
2. The – OH group of carboxylic acid is more active in chemical reactions than those in alcohols.
Types of Reaction
1. Reaction in which H of carboxylic acid is replaced/Reactions involving Cleavage of O – H bond
2. Reaction in which –OH of carboxylic acid is replaced/Reaction involving Cleavage of C–OH bond
3. Reaction involving carboxylic group as whole
1. As a weak monobasic acid
Carboxylic acid dissolves in water and dissociates to form oxonium ion by donating one H+ per molecule and carboxylate ion.
The extent to which carboxylic acid ionizes in aqueous solution is expressed in terms of Acid Ionization (dissociation) constant (Ka). The greater the ionization constant, the stronger will be the acid and vice-versa. The Ka of acetic acid is 1.7 x 10-5 which shows it to be a weak acid as compared to mineral acids for which Ka is about 108 order but acetic acid is stronger acid than carbonic acid for which Ka is 1.3 x 10-7.
The low molecular mass acids neutralize alkalis and basic metallic oxides to form corresponding Carboxylate salt (which are crystalline solids most of which are soluble in water). They react with metals like Mg to liberate H2 gas. They are strong enough to release CO2 gas by decomposing carbonates and bicarbonates. The evolution of CO2 from bicarbonates is used as to distinguish carboxylic acids from weaker acids such as phenols and alcohols (some substituted phenols also give a positive test).
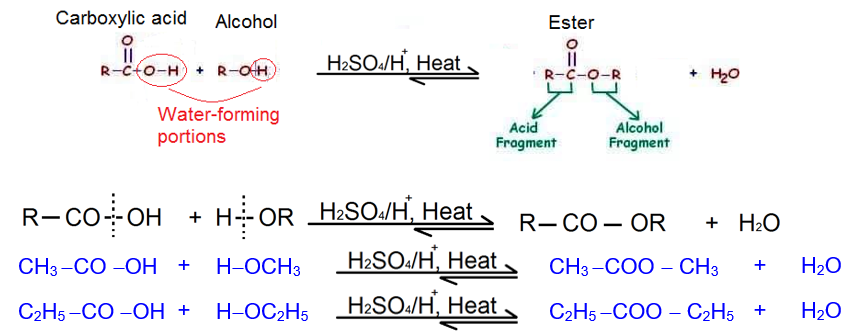
2. Esterification/Fisher esterification
The acid-catalyzed reversible condensation of an alcohol and a carboxylic acid to yield ester and water on heating is known as Fisher esterification or simply esterification. In this reaction, alcohol loses hydrogen and carboxylic acid loses –OH group. only primary and secondary alcohols not tertiary alcohols or phenols yield esters.
When esters are formed, the hydroxyl group of the acid is replaced by the alkoxides (RO–) of the alcohol (in contrast to the formation of acid salts where the acidic hydrogen is replaced by a metal).
In the esterification of tertiary alcohols and some secondary alcohols, there is fission of the R–O–H, and the yield of ester is low.
Phenols react too slowly and the equilibrium lies far to the left. Thus only primary and secondary alcohols not tertiary alcohols or phenols yield esters.
The reaction is carried out in the presence of a trace of mineral acid such as HCl or H2SO4 which takes up the eliminated water. The equilibrium usually lies on the side of the ester. The equilibrium can be shifted further to the right by using an excess of alcohol or the acid (if it the less valuable compound) or by removing the water formed through simultaneous distillation of the water with benzene or toluene.
Long chain carboxylic acids called fatty acids react with glycerol (trihydric alcohol) in the same manner to form triesters called triglycerides or triacylglycerol which commonly known as fats and oils.
The cleavage of acyl-oxygen bond has been demonstrated by Irving Roberts and Harold C. Urey (in 1938) by using isotope of oxygen. An alcohol (methanol) is synthesized that had been enriched in the mass 18 isotope of oxygen. This is reacted with an unmarked acid (benzoic acid) in which all the oxygen atoms are of the isotope of mass 16 (16O). The analysis of ester product in a mass spectrometer shows that all the 18O that was originally present in alcohol, is now present in the ester and none in the water. The results of the Robert-Urey experiment tell us that the C–O bond of the alcohol is preserved during esterification. The oxygen in water must come from the carboxylic acid. (The analysis of isotopic enrichment was performed by mass spectrometer).
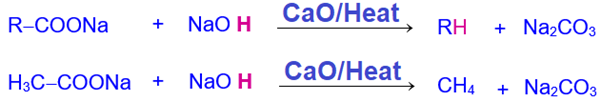
3. Decarboxylation
When sodium carboxylate(obtained by neutralizing carboxylic acid with NaOH) is heated with soda lime (a mixture of quick lime and concentrated NaOH solution), it undergoes decarboxylation losing one carbon atom from the chain as CO2 which is absorbed by NaOH , the active ingredient of soda lime .
4. Reaction with phosphorus halides and thionyl chloride
Carboxylic acids readily react with thionyl chloride in the presence of solvent pyridine or phosphorus trihalides or phosphorus pentahalides to form Acid Halide or Acyl halide or Alkanoyl halide. Thus acid halide may be regarded as Acid derivative of Carboxylic acids.
Reactions of Acid Halides
1. Reaction with alcohols and formation of esters
2. Reaction with sodium alkoxide and formation of esters
3. Reaction with ammonia and formation of amides
4. Reaction with sodium carboxylate and formation of acid anhydride
1. Reaction with alcohols and formation of esters
Acid halides react with alcohols to give esters.
2. Reaction with sodium alkoxide and formation of esters
Acid halides react with sodium alkoxide to give esters.
3. Reaction with ammonia and formation of amides
Acid halides react with ammonia to give acid amide.
4. Reaction with sodium carboxylate and formation of acid anhydride
Acid halides react with sodium carboxylate to form acid anhydride. [They are so named because they may be regarded to be derived from two molecules of acid by elimination of water molecule.]