Chemistry and its Branches
Definition of Chemistry
Science can be defined, as a never-ending search
for truth and it is the system of knowledge, which is based on a set of facts,
our understanding of those facts and verification of those facts by experiments.
Thus science is the study of universe that deals with matter, energy, life and
various aspects of life.
Chemistry is the "scientific study of matter, its properties,
and interactions with other matter and with energy".
“Chemistry is the branch of science which deals
with the study of composition, structure, properties (physical and chemical) and transformation of matter along with the chemical changes that occur in it.
It involves the study of physical
and chemical changes that matter undergoes and the energy changes accompanying these changes. It also deals with laws and principles which govern these
changes.”
The word chemistry is
derived from the word “Kheem”, an
old name of Egypt due to black colour of Egyptian soil. But some experts believed
that the word chemistry came from the word “Chyma”
meaning melt or cast. As the time passed on the word changed to Al-kimyain in Arabic and then to
chemistry in English.
Branches
of Chemistry
The
complete understanding and mastery over vast scientific knowledge is almost
impossible. To facilitate the study of science, it has been subdivided into
different disciplines. Chemistry, being a vast discipline of science has also
been divided into a number of branches to facilitate its study. As our universe
is an integrated unit so is its knowledge. There are no clear-cut boundaries
between these branches. Chemists have made these divisions for the sake of
their own convenience. All these branches of chemistry must deal with each
other one way or another. If they did not work in unison it would be impossible
for these chemistries to perform the functions we need for experiments. Thus
all these branches of chemistry overlap each other. For example, one would not
be able to measure the change in an organic or inorganic substance without
knowing how to use analytical chemistry or without some proficiency in
analytical chemistry. Chemistry can be divided into branches according to
either the substances studied or the types of study conducted. The primary
division of the first type is inorganic chemistry and organic chemistry and
divisions of the second type are physical chemistry and analytical chemistry.
The chemistry has been divided into
following nine main branches:
Physical
Chemistry
It is the branch of chemistry that deals with the physical properties of substances and
their dependence on chemical bonding. It deals with the forces and laws and principles governing the combination of atoms
and molecules. It is especially concerned with energy changes in physical and chemical processes.
Inorganic
Chemistry
It is that branch of chemistry that deals with the study of all
elements and their compounds generally obtained from non-living or mineral
origin. The detailed study of
carbon compounds (or organic compounds) especially carbon-hydrogen compounds
(hydrocarbons) and their derivatives are avoided in inorganic chemistry.
However some carbon compounds like metal carbonates (CO32¯),
bicarbonates (HCO3¯), cyanides (CN¯), thiocyanates (CNS¯), cyanates
(CNO¯), carbides (C4¯), and oxides of carbon (CO and CO2)
are studied in inorganic chemistry.
Organic
Chemistry
With the exceptions of CO, CO2,
metal carbonates, bicarbonates, cyanides, thiocyanates, cyanates and carbides, organic chemistry is the study of
essentially all carbon compounds generally obtained from living organisms. In
fact, it is the chemistry of hydrocarbons
(carbon-hydrogen compounds) and their
derivatives. Most of the
consumer products are organic in nature.
Biochemistry
It is the branch of chemistry that deals with the compounds and their reactions (metabolism)
in living organisms (i.e. in plants and animals). Biochemistry is the
backbone of medical science.
Industrial
Chemistry
It is the branch of chemistry that deals with the study of different chemical processes involved in
the industry for the large scale manufacture of synthetic
products like cement, glass, paper, fertilizers, soaps, detergents, medicines,
plastics, paints, soda ash, caustic soda etc. Industrial chemistry helps us in the manufacturing of the
industrial products and their uses. It
is the application of chemical knowledge in technology and industry for
preparation of industrial products on large scale.
Nuclear Chemistry
It deals with the changes occurring in the nuclei of atoms
accompanied by emission of radiation. It also deals with the characteristics of
radioactive processes both natural
and artificial and atomic energy
generated there.
Analytical
Chemistry
It deals with the methods and
techniques used to determine the kind and quantity of various components in
a given substance.
Environmental Chemistry
It is the study of the interaction of various chemical materials
and their effects on human environment. Pollution, personal hygiene and health
hazards are important aspects of environmental chemistry.
Polymer Chemistry
It deals with the study of polymerization and the products obtained
through the process of polymerization called polymers such as plastics like
polyvinyl chloride (PVC), papers, synthetic fibers etc.
Importance of Branches of Chemistry
Chemistry also help us to understand the nature of our environment
and about ourselves.The theories of chemistry illuminate our understanding of
the material world from tiny atom to giant galaxies.
Chemistry plays a vital role in the modern world. It has not only changed our standard of
living but also has improved health conditions. Every branch of chemistry has
its own importance in human life.
1. Biochemistry is the backbone of medical
science.
2. Industrial chemistry helps us in manufacturing
of industrial products
3. Environmental chemistry tell us that how one
can protect its environment from environmental hazards.
4. Analytical chemistry is important to
understand the composition of compounds, quality of products, analysis of
biological samples (urine, blood, milk etc.)
5. Nuclear chemistry gives atomic energy that
can be used in various fields. It also provides us Radioisotopes for the
treatment of many diseases such as cancer.
Chemistry and Society
There are three significant reasons
to study chemistry:
Firstly; chemistry has important
practical applications in the society.
Second; chemistry is an intellectual
enterprise, a way of explaining our material world.
Third, chemistry figures prominently
in other fields such as in biology, the advancement of medicines.
The role of chemistry in the prevailing society is of enormous
benefits. We are familiar with many chemicals, which have become part and
parcel of our daily life. Chemistry has deep influence on our daily living. It
matters with the protection of environment, providing our everyday needs of
food, clothing and shelters, giving pharmaceutical chemicals that enhance our
health and prolong our lives. For instance, drugs or medicines to fight
diseases, pesticides to protect our health and crops, fertilizers to grow our
crops for abundant food, food, plastic, soap, detergents, cosmetics, cement,
glass, synthetic fibres to provide comfort and variety in clothes, explosives
are the major gifts of chemistry. For
example:
1. Chlorine has
become an essential commercial chemical and this single element is used for
producing more than one thousand chlorine compounds of great industrial
importance, such as polyvinyl chloride (PVC) as plastics for pipes. Other
chlorine compounds are employed as bleaching agent, disinfectant, solvents,
pesticides, refrigerant, flame retardant and drugs. Chlorine itself is used to
kill all pathogenic (disease-causing) organisms, which causes cholera, typhoid
fever and dysentery (water–borne diseases transmitted through impure drinking
water).
2. Fluoride
compounds such as sodium fluoro phosphate (SnF2.Na2PO4.F)
and NaF in our tooth pastes help
to protect and control tooth decay and it is a great beneficence of chemistry
on the society.
Landmarks in the History of Chemistry
(Historical Back Ground of Chemistry)
Chemistry is as old as human civilization. Over the centuries
chemistry has undergone remarkable progress. Chemistry from the very beginning
was used in pottery making, glass making, dyeing and in metallurgy. The development
of chemistry can be divided into following periods:
1.
The Greek
Period
2.
Muslim or
Al-chemical Period
3.
The Modern period
The Greek Period
Scientist of Greek period
1.
Plato (347- 428 B.C)
2.
Aristotle (322- 384 B.C)
3.
Democritus (357- 460 B.C)
4.
Socrates
Contribution of Greek period
1. The Greek philosophers contributed a lot in
number of small way to the early development of chemistry. [The Greek philosophers were the first to
develop ideas relating to chemistry].
2. They introduced the concept of elements,
atoms, shapes of atoms and chemical combination (reactions). The Greek
philosopher Democritus in the 5th century put forward the idea that
matter consisted of very small indivisible particles, which he named Atomos (nowadays called atoms).
3. They believed that all matter was derived
form four elements (components) i.e. earth, air, fire, and water. They also
believed that the combination of these materials could produce new materials.
According to them fire was hot and dry,
earth was dry and cold, water was cold or hot and wet and air was cold or hot and wet.
4. The Romans developed and improved
metallurgical processes and enamellings of pottery.
Unfortunately, all these
developments were empirical (experimental) and achieved by trial and error
method without the basis of any systematic study. Greeks were basically
philosophers believing in theoretical ideas and not in experimental
confirmation of their ideas and thus they presented chemistry and science as a
theoretical subject. Therefore, Chemistry could not develop and flourish during
this period. [Thus many of the Greek principles proved wrong afterward for the
same reason].
The Muslim
Period
Scientists or
Al-chemists of Muslim period
1.
Jabir Ibne-
Haiyan (721-803 A.D)
2.
Abu Baker Al-Zakaria
Al-Razi (862-930 A.D)
3.
Al-Beruni (973-1048 A.D)
4.
Abu Ali
Ibne-Sina (980-1037 A.D)
Achievements of Muslim Period
1. The period form 600-1600 A.D. in the history
of chemistry is known as the period of
alchemist. This is the period where foundation of modern science took
place. The Muslim scientists made rich contributions to various branches of
science. In-fact Muslims are the Torch Bearers of modern science.
2. They made
use of scientific methods and thus they treated and presented chemistry as an
experimental science.
3. The alchemists developed and used such
laboratory equipments such as funnels, beakers, balances, scale for weighing,
crucible for melting metals, retorts for distillation etc.
4. They discovered fundamental methods of chemistry,
like calcinations, distillation, sublimation, filtration and fermentation.
Major achievements of Jabir Ibne-Haiyan
1. He was considered as first experimental or
practical chemist. He is known as the Father of chemistry.
2. He invented chemical methods like sublimation,
fractional distillation.
3. He invented experimental methods for the
preparation of nitric acid, hydrochloric acid and white lead.
4. He developed methods for extraction of metals
and dyeing of clothes
Major
Achievements of Al-Razi (864-930 AD)
1. He was a
physician, alchemist and a philosopher.
2. He prepared
alcohol (C2H5OH) by fermentation of sugar and starch.
3. He divided
the substances into living and non-living origin.
4. He was an
expert surgeon and was the first to use opium as anaesthesia.
Major Achievements of Al-Beruni (973-1048 AD)
1. He
determined densities of different substances.
2. He
contributed in physics, mathematics, geography and history.
The Modern Period
The modern chemistry began in 17th
and 18th centuries. The beginning of 19th century is
marked by Dalton’s atomic theory and since then, the advancement of chemistry
became very rapid. The 20th century is characterized by outstanding
achievements in determining structure of atoms and molecules, understanding of
biochemical basis of life, the development of chemical technology and the mass
production of chemicals and industrial products.
Scientists of Modern Period
Following are the contributions made by different scientists.
1. Robert Boyle ................Was regarded as the father of modern chemistry.
2. J. Black ......................Made a study of carbon dioxide.
3.J. Priestly.....................Discovered oxygen, hydrogen chloride and sulphur dioxide.
4.Scheele........................Discovered chlorine.
5.Cavendish ...................Discovered hydrogen.
6.Lavoisier..........................Discovered that oxygen constituted about 1/5th of air.
7.John Dalton ............Put forward atomic theory of matter and the concept of atomic weight.
8.Gay-Lussac............Found out relative atomic and molecular masses of many substances.
9.Avogadro...............Found out relative atomic and molecular masses of many substances.
10.J.J. Berzelius ........Introduced the idea of symbols, formulae and chemical equations
11.Mendeleev............Published the periodic table of the elements.
12.Arrehenius ...................Put forward his ionic theory of ionization.
13.M. Faraday.......Discovered the laws of electrolysis.
14.J.J. Thomson.....Discovered electrons
15.Henry Becquerel........Discovered Radioactivity
16.Madam Currie.......Established radioactivity.
17.Ken Rutherford....Discovered nucleus and put forward atomic model.
18.Neil Bohr...............Improved Rutherford’ atomic model
19.Henry Moseley............Discovered atomic number that led to the development of modern periodic table.
20. Henry Moseley............Put forward theory of unification.
Scientific Approach in Chemistry
Definition of scientific method
The scientific method is the systematic and cyclic process by which
scientists, collectively and over time; endeavor to construct an accurate (that
is, reliable, consistent and non-arbitrary) representation
of the world. Thus a method of investigation involving observation and
theory to test scientific hypotheses is called a scientific method. A
scientific method or process
is considered fundamental to the scientific
investigation and acquisition of new knowledge
based upon physical evidence.
Science is not
only an integrated knowledge of physical or biological phenomenon but also the
methodology through which this knowledge is collected. In science the facts are
gathered through observations and experimentations and then theories or laws
are deduced.
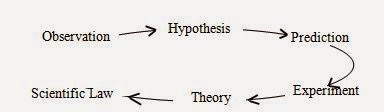
Steps
of Scientific Method
[The scientific method is a cyclic process which involves observation of
a phenomenon to collect facts thereby making hypothesis for prediction about
that phenomenon then experimentations are carried out for testing the
prediction thereby establishing a theory which if proved true then acquire the
shape of law].
The scientific method includes
following steps:
1. Observations
Observation is
basically the watching something and taking note of anything it does. In other
words observation is the process of watching, noticing and recording of
a natural phenomenon. We make observations
of natural processes and collect data about them. The observations are made by
the five
senses of man. Observation is a basic tool to go forth for elaborating
a phenomenon but it may vary from person to person according to his own skill
of elaboration.
2. Hypothesis:
This is an educated guess based upon observations. It is a rational explanation of a single event
or phenomenon based upon what is observed, but which has not been proved. Most
hypotheses can be supported or refuted by experimentation or continued
observation. A hypothesis is an educated
guess consisting of a general assumption or a proposed explanation that
results from research and prior observations of a natural phenomenon or an
observable phenomenon. However, a hypothesis has not been tested.
It generally relates to one specific idea or phenomenon.
A hypothesis is a provisional or working explanation, assumed true only to guide
experimentation or for the sake of argument.
In scientific method, the facts
collected through observations are carefully arranged and properly classified
correlating the knowledge thus acquired with the previous knowledge. Then
scientist tries to think of a tentative solution to explain the observed
phenomenon. This tentative explanation is called a hypothesis.
1. Prediction
It is the third step of scientific method.
The
inference based on observed facts is called prediction. It gives the detailed explanation about the phenomenon on the basis of gathered
facts and collected by observation
and hypothesis.
2. Experiment
The experiment is a cornerstone in empirical approach to knowledge. An
experiment is an integrated activity performed under suitable conditions with
specially designed measuring and observatory instruments to verify (or falsify)
or to test the validity of a hypothesis. Stated differently, an
experiment is a process that helps in testing the facts collected by
observation, hypothesis and predictions. [The verification of
hypothesis by experiment helps to improve the reliability of known facts. Even
unauthentication of hypothesis by experiment still gives valuable information
that can be used to deduce other results].
3. Theory
[A hypothesis
is an educated guess that results from research and prior observations of a
natural phenomenon. However, a hypothesis has not been tested. When consistency
is obtained through repeated experimentation, the hypothesis becomes a theory
and provides a coherent set of propositions, which explain a class of
phenomena.]. A theory is a well established explanation or a scientifically acceptable idea or principle
to account for a phenomenon. In other words the hypothesis that is
supported by repeated experimentations and proved to be true is called a
theory. A theory is then a framework within which observations are explained
and predictions are made. Thus a theory is a thoroughly tested model that
explains why experiments
give certain results. [The main difference between theory and a law is that a
theory is an explanation for the pattern in the data or phenomenon and explains
how it happens, often by using an analogy or metaphor while a law is just a
description of a pattern in the data and merely shows what happens, without any
explanation].
4. Scientific Law
A theory that repeatedly gives the same
results after experimentation offering correct explanation of scientific facts
and from which valid predictions can be made is known as Scientific Law or
Scientific Principle. Thus a law is a theory that has passed the test
of time and is generally accepted as truth. A Scientific Law is
an accepted scientific principle taken to be correct and universally
applicable. However, not all hypotheses and theories pass
successfully to become scientific laws. Some hypothesis or theories may sound
very convincing and are well supported by mathematical calculations but are
very difficult to prove experimentally. This is invariably due to the material under
consideration or the lack of the suitable working equipments. A typical example
is of Avogadro’s law (or hypothesis) that has not been proved conclusively and
yet it is accepted as Law.