Extraction
of Copper from Sulphide Ore
Ore used and Type of Metallurgy
Copper is extracted
mainly from sulphide ore, copper pyrite (Chalcopyrite), CuFeS2 (CuS
+ FeS or Cu2Fe2S4 i.e. Cu2S + Fe2S3)
which contain about 34% (6% in book) copper by Pyrometallurgy (Dry Process).
Main steps of Metallurgy
Extraction
of Copper from sulphide ore involves the following steps:
1. Crushing of Ores into Powdered Form
The big
blocks of ores are crushed into small pieces and then finally powdered.
2. Concentration of Crushed Ore
Ores are often
contaminated with non-metallic and rocky impurities like sand (quartz), clay,
mica, lime stone etc which are called gangue or matrix. The removal of unwanted
impurities from the ore before metallurgical process is called Concentration or benefication or ore
dressing. Copper pyrite is a low grade copper ore containing about 34%
copper along with impurities such as sand, clay, lime stone etc.
The
powdered ore is concentrated by froth
floatation process (Selective Wetting) which is based upon preferential wetting of surfaces by
liquids on account of the surface
tension forces.
The powdered or
crushed ore is concentrated by froth floatation process, in which the crushed
ore is mixed with a mixture of Pine Oil
or creosote oil and water and then thoroughly agitated with a blast of air.
The ore is miscible (get wetted) with oil forming froth and rises to the top from
where they are skimmed off and dried to get concentrated enriched ore whereas
the gangue particles (impurities) are wetted by water and settle at bottom.
Copper content is increased to 25-30% in this way.
3. Roasting
Roasting
is a process in which the concentrated ore is strongly heated in a current of
excess of air below the melting point of ore in the hearth of a large flat
Reverberatory Furnace. Roasting not only dries the ore but also bring about
following changes:
(i) Impurities like C, S, P, As,Sb in the ore are
removed as their volatile oxides. It also removes organic matter
|
S
|
+
|
O2
|
¾¾¾¾¾®
|
SO2
|
|
4Sb
|
+
|
3O2
|
¾¾¾¾¾®
|
2Sb2O3
|
|
4As
|
+
|
3O2
|
¾¾¾¾¾®
|
2As2O3
|
(ii)Copper
pyrite (ore) is converted into a mixture of Cuprous Sulphide and Ferrous Oxide.
Some FeS present in the ore remains unreacted. At the end of roasting, the ore
has been now contained Cu2S, FeO and FeS.
4. Smelting (Formation of Molten Matte)
Smelting
is the process of reduction of oxide of ore by reducing agent (like coke) under
such conditions that metal is obtained in molten state.(In copper extraction,
smelting serves to remove gangue).
A charge
consisting of Roasted Ore, powdered Coke (reducing agent) and sand (flux) is strongly
heated in a Water Jacketed Blast Furnace and a blast of hot air is blown at the
lower part of furnace through tuyeres. During smelting coke burns releasing heat
of combustion which serves to keep the charge in molten state.
The
Ferrous oxide present in ore combines with sand (flux) to form fusible ferrous
silicate (slag) which being lighter rises to the top and is withdrawn from the
upper hole.
The
molten mixture of Cu2S and FeS is left behind after smelting which
is called Molten Matte or Course Metal wchih contains about 45% copper. It
being heavier, forms the lower layer and is withdrawn from the lower hole
periodically.
5.
Bessemerisation (Formation of Blister Copper)
The reduction
of molten matte is carried out in a pear-shaped stainless steel furnace called
Bessemer converter which is lined inside with MgO also provided with tuyers.
The
molten matte is heated in Bessemer converter by blowing hot blast of air
through pipes called Tuyers with more silica. The following changes occur in
the converter:
(i) Air oxidizes most of FeS to FeO by direct
oxidation which is removed as ferrous silicate (slag) by combining with silica.
FeS is also converted into FeO by reacting with Cu2O (formed by
partial oxidation of Cu2S).
(ii) After
the iron has been removed, hot blast of air partially oxidizes Cu2S
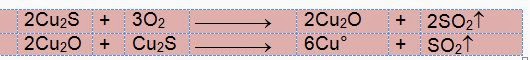
into Cu2O (leaving some unreacted Cu2S). Now Cu2O
and remaining Cu2S reduce each other (Auto or self reduction)
without any external reducing agent to give metallic copper in molten state.
Blistered Copper
The molten metal produced is now cooled to solidify in
sand moulds, the dissolved and hidden SO2 gas escapes out, produces
blisters (bubbles) on the surface of solid metal, so copper obtained is called
BLISTERED COPPER which is 98-99% pure.
Since the reaction is exothermic, the heat liberated
keeps the crude copper in the molten state. The completion of the reaction is
indicated by the appearance of green flame produced by the vapourization of
copper.
6. Electrolytic Refining of Blistered Copper
Blistered copper
contains impurities mainly Ag, Au, Pt, Ni, Zn, Fe, As, Pb, As etc. Hence for
electrical purposes, blister copper obtained after bessemerization is refined
electrolytically to get copper of high purity (99.95-99.99).
The
refining of Blistered Copper is carried out in a large Lead-Lined Tank using a
series of anode of thick impure blister copper which places alternately with a
series of cathodes of thin plates of 100% pure copper (The cathodes are coated
with graphite so that the deposited pure copper may be removed easily). These
electrodes are suspended in an electrolytic solution of CuSO4 (15%) acidified
with traces of dilute H2SO4 (5%).
On
electrolysis, the atoms of anode (impure copper metal and other active metals
such as Fe, Zn, Bi etc) are oxidized to their corresponding cations and pass
into solution. The impurities of less active metals such as Ag, Au etc are not
oxidized and settle down just below the anode as anode mud. The current
of 1.3–1.5 volt is used for electrolysis which helps to deposit only copper on
cathode leaving behind other metal ions in solution. The electrolytically
refined copper is 100% (99.99%) pure. With the deposition of copper, cathode
grows thick and anode becomes thin due to dissolution of the metal.
Electrolytic Reactions





Nice
ReplyDeleteThanx Shaks. Keep visiting my blog.
Deleteits very helpful for me
ReplyDeleteThanks .. nice information !
ReplyDelete