Carbohydrates and its biomedical Importune & types
Carbohydrates
are natural macromolecules including a large number of relatively
heterogenous compounds containing C, H and O and are the most
abundant biomolecuels on the earth. They are commonly called sugars or
saccharides (Latin; saccharum meaning sugar).
Carbohydrates are defined as the polyhydroxy
(polyhydric) aldehydes or polyhydroxy ketones or other compounds (large
polymeric compounds) that yield these derivatives (i.e. polyhydroxy aldehydes
or ketones) upon hydrolysis. Carbohydrates are regarded as the Hydrates of
Carbon because the ratio of O and H as same as it is in water (i.e. in the
ratio of 2:1). Their general formula is Cx(H2O)y.
glucose, galactose, fructose,
sucrose, lactose, maltose, starch, cellulose, glycogen etc are some typical
carbohydrates.
CHO
|
H – C – OH
|
CH2OH
|
CH2OH
|
C = O
| CH2OH
|
CHO
|
H – C – OH
|
HO – C – H
|
H – C – OH
|
H – C – OH
|
CH2OH
|
CHO
|
H – C – OH
|
HO – C – H
|
HO – C – H
|
H – C – OH
|
CH2OH
|
CHO
|
HO – C –H
|
HO – C – H
|
H – C –
OH
|
H – C – OH
|
CH2OH
|
CHO
|
C
= O
|
HO – C – H
|
H – C – OH
|
H – C – OH
|
CH2OH
|
Glyceraldehyde
|
dihyroxyacetone
|
D-glucose
|
D-galactose
|
D-mannose
|
D-fructose
|
Carbohydrates
are regarded as the Hydrates of Carbon because many of them contain H and O in
the same proportion as in water (i.e. in the ratio of 2:1) and that is why they
may be represented by a general formula Cx(H2O)y
or CxH2yOy (where x = 3 or some large number).
However, this definition proved to be erroneous because:
1)Some compounds such rhamnose (C6H12O5),
Menitol (C6H14O6) etc are definitely carbohydrates
but their formulae do not conform to the
general formula Cx(H2O)y.
2) All compounds conforming to the general
formula are not necessarily carbohydrates e.g. formaldehyde,
HCHO (CH2O), acetic acid, CH3COOH [C2 (H2O)2]
etc.
Bio-medical Importance of Carbohydrates
1.
|
Regulation
of metabolism
|
(Ribose,
C5h10O5
and deoxyribose, C5H10O4)
|
2.
|
Genetic
regulation
|
(DNA
and RNA; hereditary carriers)
|
3.
|
Dietary
Source
|
(Sugar,
Starch, glucose)
|
4.
|
Energy
Source
|
(Glucose)
|
5.
|
Storage
Function
|
(glycogen)
|
6.
|
Extra Energy source for animals
|
(Cellulose)
|
Classification of Carbohydrates on
the basis of Taste
1. Sugars; Sweet, crystalline and water-soluble e.g Glucose, fructose 2. Non- sugars; Tasteless, amorphous and water-insoluble e.g. Starch, cellulose |
(1). Sugars
The
crystalline water soluble carbohydrates which are sweet in taste are called
sugars. All monosaccharides and disaccharides are sweet in taste and hence
monosaccharides and disaccharides are commonly called sugars. e.g.
Glucose, fructose, sucrose, maltose etc.
(2). Non-sugars
The amorphous
water insoluble tasteless carbohydrates are called non-sugars. All polysaccharides
are non-sugars. e.g. starch, cellulose etc. are non-sugar carbohydrates.
Classification of Carbohydrates on
the basis of Free Aldo or Keto Group
1.
|
Reducing
Sugars
|
;
|
Contains free aldo or keto group
|
e.g.
|
glucose, galactose, fructose,
maltose
|
2.
|
Non-reducing sugars
|
;
|
Lacks
free aldo or keto group
|
e.g.
|
Sucrose ,Starch, cellulose
|
Reducing Sugars
Reducing
Sugars are those sugars that contain free aldehydic or ketonic group (along with
adjacent – OH group) and they can reduce mild oxidizing agents like Benedict’s
reagent [alkaline solution of citrate complex of copper (II)], Fehling’s
Solution [alkaline solution
of tartarate complex of copper (II)] or Tollen’s reagent [ammonical silver
nitrate solution]. All monosaccharides and all disaccharides except sucrose are
reducing sugars. E.g. glucose, galactose, fructose, maltose, lactose.
Non-reducing sugars
Non-reducing
Sugars are those sugars, which do not contain free aldehydic or ketonic group,
and they cannot reduce mild oxidizing agents like Benedict’s reagent, Fehling’s
Solution or Tollen’s reagent. e.g. One disaccharide (e.g. Sucrose) and all
polysaccharides (e.g. starch, cellulose etc)are non-reducing sugars.
Classification of Carbohydrates Based
on Molecular Structure or Complexity
Based on
complexity of molecular structure, behaviour on hydrolysis and number of carbon
atoms or the number of simple sugar units present in their molecules,
carbohydrates are classified into following three major classes:
1.
|
Monosaccharides(Simple sugars)
|
(Non-hydrolyzable
reducing sugars; containing 3-9 carbons atoms with general formula CnH2nOn or
(CH2O)n )
|
2.
|
Oligosaccharides (e.g.
Disaccharides)
|
(hydrolyzable reducing sugars (except
sucrose); giving 2-10 simple sugars)
|
3.
|
Polysaccharides
|
(hydrolyzable
non-reducing tasteless amorphous non-sugars; which are macromolecular high
molecular mass natural polymers giving more than 10 simple sugars on
hydrolysis)
|
Monosaccharides
1. These
are the simplest carbohydrates and are non-hydrolyzable simple sugars
that cannot be further hydrolyzed into simpler carbohydrate units consisting of
aldoses or ketoses. [The monosaccharides having aldehydic group are
aldoses (e.g. glucose) while monosaccharides containing ketonic group are
called ketoses (e.g. fructose). The aldehydic group being monovalent is always
present at the end of the carbon chain while ketonic group being divalent can
be present anywhere in the carbon chain but in naturally occurring monosaccharides
the keto group is present at carbon number 2].
2. They
are also known as reducing sugars because they have free sugar (i.e. aldehydic
or ketonic) group, so they can reduce Benedict’s Reagent (citrate complex of Cu2+
ions), Fehling’s Solution (tartarate complex of Cu2+ ions) and
Tollen’s reagent (ammonia complex of Ag+ ions).
3. They
have the general formula CnH2nOn or (CH2O)n
where n is the number of carbon atoms ranges 3-9 (3-10). Hence they are named
as trioses, tetroses, pentoses and hexoses depending upon the number of 3, 4, 5
and 6 carbon atoms respectively. e.g. aldopentoses (ribose, arabinose, xylose)
aldohexoses (e.g. glucose, galactose, mannose) ketopentoses
(ribulose), ketohexoses (e.g fructose or levulose etc.) [Ose is the suffix for aldo
sugar and Ulose is the suffix for keto sugars].
4. Sugars
with five carbon atoms (pentoses) or six carbon atoms (hexoses) are more stable
as cyclic structures than as open
chain structures.
5. Among
hexoses glucose, galactose, mannose and fructose are important from nutritional
and muscular physiological point of view. Among pentoses ribose, arabinose
(obtained from Gum-Arabic) and xylose (wood’s component) and deoxyribose play
an important part in human metabolism.
Oligosaccharides
1. These
are carbohydrates which are formed when 2-10 (2-9) monosaccharide units
condensed together through glycosidic linkage by the loss of water molecule.
Conversely the carbohydrates which give 2-10 (2-9) simple sugar
(monosaccharide) units on hydrolysis by water (in the presence of an acid or by
enzymes). Among oligosaccharides, the most important is disaccharides are important.
2. Disaccharide
is formed by the joining (condensation) of two molecules of same or different
monosaccharides units through glycosidic linkage by giving out a water molecule
and thus on hydrolysis disaccharide yield two units of simple sugars. e.g. Maltose,
lactose, sucrose. They are commonly known as saccharose having the general
formula C12H22O11.
3. Except
sucrose, all disaccharides are reducing sugars.
Polysaccharides
Polysaccharides
are carbohydrates of high molecular mass that yield more than 10
monosaccharides units upon hydrolysis and are made up of long chains of many monosaccharides
units joined to each other through glycosidic linkage in a linear or branched
structure by the process of polymerization. They are non-sugars,
water-insoluble, tasteless amorphous solids. Their principal functions are food
storage (energy storage compounds) and structural building of cells. e.g. Starch,
cellulose, glycogen (animal starch), chitin.
Glycosidic Linkage
Glycosidic
linkage is the characteristic of oligosaccharides (disaccharides etc.) and
polysaccharides as it joins two or more glycosides (monosaccharides). [Disaccharides
are formed by a condensation reaction between two monosaccharides accompanied
by the elimination of a water molecule].
A
Glycosidic Linkage is one in which a carbon atom is joined to two oxygen atoms
through single bond (i.e. -O - C - O -) which differs from ether linkage (> C - O - C< ) in that, in the latter, oxygen is joined to two
carbon atoms while in glycosidic linkage one of the carbon atom is attached to
two oxygen atoms.
Formation of glycosidic linkage between hydroxyl groups of the
hemiacetals of a–glucose and b– glucose units at C–1
and C–4 condense to form a disaccharide called maltose. This O - C - O linkage is called a,b-1:4-glycosidic
linkage
There are
following types of glycosidic linkages:
1.
|
a,b-1:4-glycosidic
linkages
|
(in maltose)
|
[b-glucose and a-glucose]
|
2.
|
b-1:4-glycosidic linkages
|
(in lactose)
|
[b-glucose and b-galactose]
|
3.
|
a,b-1:2-glycosidic
linkages
|
(in sucrose)
|
[a-glucose and b-fructose]
|
D-Sugars
and L-Sugars
The D and L-forms
of an isomer (enantiomer) is related to standard glyceraldehydes whose two
forms are arbitrarily assigned the absolute configurations. The glyceraldehyde
in which penultimate asymmetric carbon atom has hydroxyl group to the right and
hydrogen to the left is designated as D-form while the glyceraldehydes in which
penultimate asymmetric carbon atom has hydroxyl group to the left and hydrogen
to the right is designated as L-form. If the configuration at the penultimate
(the lowest or farthest from the top) asymmetric carbon atom of a compound is
related to D-glyceraldehyde, it is designated as D-form and it is related to
L-glyceraldehyde, it is designated as L-form. (The symbols D and L signify the
arrangement of atoms or groups in space and are not related to the property of
the molecule to rotate the plane of polarized light towards right or left).
CHO
|
H – C – OH
|
CH2OH
|
CHO
|
HO – C – H
|
CH2OH
|
D-glyceraldehyde (D-configuration)
|
L-glyceraldehyde (L-configuration)
|
( - OH group on the right side of chiral C)
|
( - OH group on the left side of chiral C)
|
In case of compounds with several
asymmetric carbon atoms like sugars, the first asymmetric carbon atom from the
bottom (i.e. penultimate chiral C) is always taken as the reference carbon atom
for absolute designations. As per convention for sugars, the carbon atom-5 is
taken as the reference atom for D-L designations.
All those sugars in which the first
asymmetric carbon atom from the bottom i.e. penultimate chiral carbon has –OH
group on right hand side are termed as D-sugars while those sugars in which –OH
group is on the left hand side at the first asymmetric carbon atom from the
bottom are referred as L-sugars. Most of the natural sugars have D-configuration.
Naturally occurring glucose is D-glucose.
CHO
|
H – C – OH
|
HO – C – H
|
H – C – OH
|
H – C – OH
|
CH2OH
|
CHO
|
H – C – OH
|
HO – C – H
|
HO – C – H
|
H – C – OH
|
CH2OH
|
CHO
|
HO – C –H
|
HO – C – H
|
H – C –
OH
|
H – C – OH
|
CH2OH
|
CHO
|
C
= O
|
HO – C – H
|
H – C – OH
|
H – C – OH
|
CH2OH
|
D-glucose
|
D-galactose
|
D-mannose
|
D-fructose
|
Dextrorotatory
and Laevorotatory Form and Mutarotation
Different forms of a compound which
resemble in their chemical and most physical properties but differ in their
optical behaviour towards the plane polarized light are called optical isomers
and the phenomenon is called optical isomerism.
The optical isomers which are
related to each other as object and its mirror image are called enantiomers.
The enantiomer (optical isomers)
which rotates the plane of polarized light to the right is called dextrorotatory
and is conventionally given a positive sign (+) and described by d. The mirror
image of enantiomer (optical isomer) which rotates the plane of polarized light
to the left is called Laevorotatory and is conventionally assigned a negative
sign (-) and denoted by l. e.g. D-glucose is dextrorotatory and hence is called
dextrose and D-fructose is laevorotatory and hence is called Levulose (Laevulose).
The spontaneous change in the
specific rotation of an optically active compound involving the dynamic
interconversion of two isomeric closed chain structures into one another via
open chain form is called mutarotation. These two closed chain isomers are
called a and b forms which differ only in the
orientation of the hydroxyl group at carbon atom-1. The closed chain structure
of an optically active compound in which –OH group is on right hand side at
carbon number 1 (anomeric or glycosidic carbon) is called a-isomer and when –OH group is on the left hand side
at carbon number 1 is termed as b-isomer. Such pairs of optical isomers differing
in the orientation of H and OH groups only at carbon atom-1 (anomeric or
glycosidic carbon) are called anomers. E.g. D-glucose exists as two anomeric
forms a-glucose and b-glucose which are separate crystalline
forms with different melting points and optical rotation but when either form
of glucose is dissolved in water, it gets converted into the other form and an
equilibrium mixture is formed of a-glucose and b-glucose together with a very small amount of open
chain form i.e.
a,D (+) glucose =
|
Open chain form D(+) glucose =
|
b,D (+)glucose
|
|||
m.p. =146°C
|
m.p.=
150°C
|
||||
36%
|
0.02%
|
64%
|
|||
+110°
|
+52.5°
|
+19.7°
|
|||
CHO
|
H –C–OH
|
HO – C – H
|
H – C – OH
|
H – C – OH
|
CH2OH
|
CHO
|
H – C – OH
|
HO – C – H
|
H – C – OH
|
H – C – OH
|
CH2OH
|
Proteins (its Definition, Importance and Types)
Definition of Proteins
All proteins yield amino acids which are generally a-amino carboxylic acids of different molecular sizes, upon complete hydrolysis. Thus, amino acids are the basic constructing units of proteins. The human body probably contains at least 10,000 different kinds of proteins. The proteins constitute 50% of the dry weight of the living cells (that is why they have given a name of proteins derived from the Greek work proteios meaning of prime importance). Proteins are present in all living organisms and are found in muscles, skin, hair, nails and other tissues that make up the bulk of the body’s non-bony structure.
Proteins (derived from Proteios meaning first) are the complex nitrogenous long chains polymeric organic macromolecules of very high molecular weight (34000- 5000000 dalton or 17500 -6,000,000 Da) which are giant linear condensation biopolymers of a-amino acid monomers (ranging from 200-6,0000) interlocked or linked together through peptide linkages (acid-amide bonds) through condensation polymerization with the elimination of water molecules. In other words, Proteins are high molecular weight organic polypeptides which yield a- amino acids upon complete hydrolysis. [The aggregation of two or more amino acids linked together through peptide bond is called Peptide. A dipeptide is a condensation product of two amino acids containing a single peptide bond while a tripeptide is a condensation product of three amino acids containing two peptide bonds. A condensation product of 10 or more amino acids is called Polypeptide]. A polypeptide of high molecular weight more than 10,000 da containing more than 200 amino acids is called protein.
All proteins contain the elements C, H, O, N and S. Other elements like P and traces of other elements such as Ca, Fe, Mn, Cu, Zn and I may be the essential constituents of some specialized proteins. e.g. Casein (milk protein) contains Ca and P, haemoglobin (iron containing protein).
Bio-medical Importance of Proteins
1.
|
Building and maintenance of body tissues
|
(Structural proteins)
|
2.
|
Catalytic Function/ Biochemical Catalysis
|
(Enzymatic proteins or Enzymes)
|
3.
|
Regulation of metabolism
|
(Hormonal proteins like insulin)
|
4.
|
Contraction of muscles
|
(Contractile proteins like Actin and myosin filaments)
|
5.
|
Storage Function
|
(Storage proteins like Lipoproteins, ferritin)
|
6.
|
Protective Function
|
(Immunal proteins like Antibodies like globulins and γ-globulins)
|
7.
|
Respiratory Function/ Oxygen carrier
|
(Haemoglobin, cytochrome, haemocyanin, myoglobin)
|
8.
|
Genetic Regulation
|
(Genetic proteins or nucleoproteins like DNA and RNA)
|
Classification of Proteins Based on Physio-Chemical Properties
Proteins are of three types:
1.
|
Simple proteins
|
;
|
Consist of only amino acids or their derivatives e.g. Albumin, globulin
|
(a) Albumins
|
(e.g. egg albumin, serum albumin, milk albumin/lactalbumin, Wheat albumin, leucosin
| ||
(b) Globulins
|
(e.g. egg globulin, serum globulin, myosin/muscle globulin, amandin in almonds
| ||
(c) Glutelins
|
(e.g.
| ||
(d) Gliadin/Prolamine
|
(e.g. hordein in barley, zein in maze corn, gliadin in wheat)
| ||
(e) Albuminoids
|
(e.g. casein & collagen in tendons, keratin in hair, horn, nails)
| ||
2.
|
Conjugated Proteins
|
;
|
Consist of simple proteins with prosthetic group e.g. Haemoglobin,
|
(a) Glyco-proteins
| |||
(b) Lipo-proteins
| |||
(c) Nucleo-proteins
| |||
(d) Phospho-proteins
| |||
(e) Chromo-proteins
| |||
3.
|
Derived Proteins
|
;
|
Degraded proteins e.g. Proteoses, polypeptides, peptides, peptones.
|
1).Simple Proteins
These are the simplest proteins which upon hydrolysis yield only amino acids and their derivatives. e.g. egg albumin, serum albumin of blood, and milk albumin (lactalbumin) etc.
2). Conjugated Proteins
These are the proteins which consist of simple proteins with non-proteinous substance called prosthetic group. e.g. Haemoglobin, Chlorophyll.
3). Derived Proteins
These are not naturally occurring proteins and are obtained by degradation of proteins by acids, alkalis, heat, enzymes or biochemical action. e.g. peptones, proteoses, polypeptides, peptides.
Definition and General Formula
Amino acids are the basic building blocks of proteins. Amino acids are bifunctional (polyfunctional) organic acids having both an acidic carboxyl group (-COOH) and a basic amino group (-NH2 or >NH) along with distinct side chain R (which is different for different amino acids). Almost all the naturally occurring amino acids are a-amino acids in which amino group is attached to a-carbon atom (relative to the carboxyl group). They have following general formula:
Where R may be hydrogen, a straight or branched chain alkyl group or an aryl group.
The amino group in amino acids may be present at any carbon atom other than that of carboxyl group containing carbon (i.e. a– carbon). Based on whether the amino group is present on the a, β or γ - carbon atom relative to the carboxyl group, amino acids are referred to as a, β and γ–amino acids respectively.
Bio-medical Importance of Amino Acids
1.
|
Protein synthesis
| |
2.
|
Hormones synthesis
| |
3.
|
Energy Source
| |
4.
|
Regulation of metabolism
|
Types of Amino Acids Based on their Acidity or Alkanity
1.
|
Neutral amino acids
|
;
|
contain one –NH2 & one –COOH group.
|
e.g.
|
Glycine, alanine, Valine, Leucine
|
2.
|
Acidic amino acids
|
;
|
contain more than one –COOH groups.
|
e.g.
|
Aspargine, Glutamine
|
3.
|
Basic amino acids
|
;
|
contain more than one –NH2 groups
|
e.g.
|
Lysine, Arginine.
|
Essential Amino Acids and Non-essential Amino Acids
About 20 amino acids have been identified as the constituents of most of the animal and plant proteins. Out of 20 amino acids which are required for protein synthesis, the human body can synthesize only 10 and such amino acids are called Non-essential amino acids. e.g. Glycine, alinine, proline, aspartic acid, glutamic acid, tyrosine, serine, cysteine, aspargine.
The ten amino acids that are not synthesized by human body and hence are needed to be provided in the diet for proper health and growth are called Essential Amino Acids. [They are 10 for infants and 8 for adult human being]. They include Valine, Leucine, Isoleucine, Lysine, Methionine, Threonine, Arginine, Tryptophan, Phenylalanine, and Histidine (last two are needed for infants).
Zwitterion
The dipolar but overall electrically neutral charged ion with positive as well as negative ends within the same molecule is termed as Zwitterion (German; two ions). The dipolar ionic structure is also called internal salt. All a–amino acids exist largely as dipolar ionic forms or Zwitterions in solution that is formed when the proton goes from the acidic carboxylic group (on ionization) to basic amino group (Lewis base).
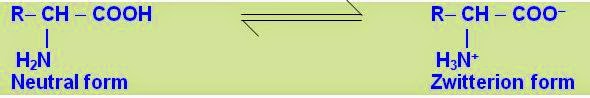 | |||||
Due to the Zwitter ions form, amino acids are soluble in water but insoluble in organic solvents. The Zwitterion formation makes amino acids amphoteric and allows them to donate or accept proton from the medium or solvent in which they are dissolved.
Peptide Linkage
The acid-amide (-CO -NH -) bond through which amino acids are linked in proteins by eliminating a water molecule is called Peptide Linkage. This linkage is formed by the removal of a water molecule b/w an -NH2 group of an amino acid and -COOH group of another. The product formed from condensation of two amino acids containing a single peptide linkage is called Dipeptide. The product formed from condensation of three amino acids containing two peptide bonds is called Tripeptide.
Lipids and its Types
Definition of Lipids
The lipids are a group of heterogenous water insoluble non-polar organic compounds of the plant and animal origins which are soluble in Bloor’s reagent (a mixture of diethyl ether and ethyl alcohol in the ratio of 2:1) e.g. Oils, fats, waxes, butter, fat-soluble vitamins (A, D, E, K) etc.
Bio-medical Importance of Proteins
Types of Lipids on the basis of structures by Bloor
A) Fats and Oils (Triglycerides or triacylglycerols)
Fats and oils are simple lipids that are triesters of fatty acids (long chain carboxylic acids containing even number of carbon atoms) with polyhydric alcohol, glycerol. Hence they are also called Triglycerides or Triacylglycerol.
Difference b/w Fats and Oils
Fats
1. Fats are solid state esters (triglycerides) of fatty acid and glycerol at room temperature.
2. Fats usually come from animal sources.
3. Fats contain a higher proportion of saturated fatty acids in their esters.
4. Fats have low iodine number (less unsaturation).
5. Usually fats are simple glycerides in which all the three –OH groups of glycerol are esterified with same acid.
Oils
1. Oils are liquids state esters (Triglycerides) of fatty acids and glycerol at room temperature.
2. Oils come from vegetable sources.
3. Oils contain a higher proportion of unsaturated fatty acids in the in their esters.
4. Oils have high iodine number (except coconut and fish oils.).
5. Usually oils are mixed glycerides containing two or more different fatty acid parts.
B) Waxes
Waxes are naturally occurring esters of long–chain monohydric alcohols and long-chain fatty acids (C16 or greater). Thus on hydrolysis wax gives monohydric alcohol [while fat gives trihydric alcohol, glycerol]. They are water-insoluble, flexible, non-reactive, low melting solids with a waxy feeling. e.g. Bee’s wax is an ester of palmitic acid and mericyl alcohol (C30H61OH) and its chemical name will be mericyl palmitate. CH3.(CH2)14.COO.(CH2)29.CH3 OR C15H31COOC16H33, spermacetic wax C15H31COO-C16H33 (Spermaceti consists principally of cetyl palmitate (the ester of cetyl alcohol, C16H33OH and palmitic acid), C15H31COO-C16H33).
Cetyl alcohol, also known as 1-hexadecanol and palmityl alcohol, is a fatty alcohol with the formula CH3(CH2)15OH. At room temperature, cetyl alcohol takes the form of a waxy white solid or flakes. The name cetyl derives from the whale oil (Latin: cetus) from which it was first isolated.
Simple and Mixed Glycerides
When the three OH group of glycerol are esterified with the same acid, the triglyceride is known as Simple Glyceride (Simple Fat).
If two or more OH groups of glycerol are esterified with different fatty acids, then the triglyceride is known as Mixed Glyceride or Mixed triglycerides or Mixed triacylglycerols (Mixed Fats). Cooking oils and fats are mixed triglycerides containing all the three different fatty acids.
|
Fatty Acids and its Types
Fatty acids are naturally occurring non-branched aliphatic monocarboxylic acids consisting of a long saturated or unsaturated hydrocarbon chain with a terminal carboxylic group mostly obtained from hydrolysis of natural fats and oils containing even number of carbon atoms (ranges C12-C20).
Saturated and Unsaturated Fatty acids
The alkyl groups of saturated fatty acids contain C-C single bond.e.g.
1.
|
Lauric acid
|
(C11H23-COOH/C12H24O2)
|
C12H24O2
|
2.
|
Myristic acid
|
(C13H27-COOH/ C14H28O2)
| |
3.
|
Palmitic acid
|
(C15H31-COOH/ C16H32O2)
| |
4.
|
Stearic acid
|
(C17H35-COOH/ C18H36O2)
| |
The alkyl group of unsaturated fatty acids contain at least C=C double bond. e.g
1.
|
Oleic acid/9-Octadecenoic acid(C17H33-COOH/ C18H34O2)
|
CH3(CH2)7CH=CH(CH2)7COOH
| |
2.
|
Linoleic acid/ 9,12-Octadecadienoic acid(C17H31-COOH/ C18H32O2)
|
CH3(CH2)4CH=CHCH2CH=CH(CH2)7COOH
| |
3.
|
Linolenic acid/9,12,15-octadecatrienoic acid(C17H29-COOH/ C18H30O2)
|
CH3CH2CH=CHCH2CH=CHCH2CH=CH(CH2)7COOH
| |
4.
|
Arachidonic acid/5,8,11,14-Eicosatetraenoic acid
|
(C19H31-COOH/ C20H32O2)
| |
Essential Fatty acids
Essential Fatty acids are those fatty acids that our body cannot synthesize so they must be taken as diet. The function of essential fatty acids is:
ii. They prevent deposition of cholesterol in arteries and veins.
ii. They help in reproductive functions and blood clotting.
All essential fatty acids are unsaturated i.e.:
i. Linoleic acid (C17H31-COOH)
ii. Linolenic acid (C17H29-COOH)
iii. Arachidonic acid (C19H31-COOH)
Chemical Properties of Fats and Oils
1. Saponification
The alkaline hydrolysis of fats or oils on boiling with a solution of strong alkali (NaOH or KOH) to form sodium or potassium salt of long chain fatty acid (soap) along with glycerol is called Saponification. It is the reverse process of esterification of glycerol.
2. Rancidification
The development of disagreeable foul smell and unpleasant bad taste in a fat or oil caused by its hydrolysis or oxidation on exposure to warm, moist air for a long time is called Rancidification or Rancidity. E.g. when butter is left uncovered, the butter fat tributyrin is hydrolyzed by microorganisms in air producing butyric acid, which imparts unpleasant offensive odour and makes it off-taste. Rancidification is due to:
i. Hydrolysis of ester linkage to give original fatty acid of sour taste.
ii. Oxidation at double bond forming volatile aldehydes of bad odour.
3. Iodine Number
The degree of unsaturation of a fat or oil is usually measured by Iodine Number that is the number of gram of iodine consumed or absorbed by 100 gram of fat or oils. Higher the iodine number, greater would the degree of unsaturation or vice versa.
The iodine number of oils is generally higher than fats (due to higher proportion of unsaturated acids in it).
The iodine number of vegetable fats is generally higher than animal fats (except coconut and fish oil).
4. Hydrogenation of Oils
The hydrogenation of unsaturated vegetable oils such as cotton seed oil converts it into fats. Hydrogenation of vegetable oils takes place in the presence of Ni catalyst.
Vitamins, its Types, Sources and Deficiency Diseases
Vitamins are the complex organic compounds which act as a catalysts or promoters and facilitate the metabolic processes required in very small amount. They cannot be synthesized by the animals hence they must be supplied in diet. They have no food value but they are also called Accessory Food Factors b/c they regulate various physiological and chemical reactions taking place in the body. [So far 13 vitamins have been isolated. Thiamine was the first vitamin discovered].
Classification of Vitamins on the Basis of Solubility
Dr Inam Ul Haq Jazbi








Great Contribution to Chemistry, Sir.
ReplyDeleteI wish you all the best for this noble cause.
Thanx Muhammad adeel.
Delete