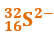Q1. The
numbers of electrons, protons and neutrons in a monoatomic specie are equal to
36, 35 and 45 respectively. Assign proper symbol.
Solution Dr Inam Ul Haq Jazbi
Given;
No. of electrons = 36,
No. of protons = 35,
No. of neutrons =45
Required;
Identify specie = ?
Identification of Specie (Z and charge)
Dr Inam Ul Haq Jazbi
Number of protons = atomic number = 35
The specie must be either Br (bromine) atom or bromine ion as it has Z = 35.
Since number of electrons given 36 which is one unit
greater than the number of protons, hence the given specie must be anion with
1- charge.
Charge on specie = Z – number of
electrons = 35 – 36 = 1-
Identification of Mass number (Z + N)
Dr Inam Ul Haq Jazbi
Atomic mass number (A) = Z + N = 35 + 45 = 80
Result
Hence the specie is
Q2. The
numbers of electrons, protons and neutrons in a monoatomic specie are equal to 18,
16 and 16 respectively. Assign proper symbol.
Solution Dr Inam Ul Haq Jazbi
Given;
No. of electrons = 18,
No. of protons = 16,
No. of neutrons =
16
Required;
Identify specie = ?
Identification of Specie (Z and charge)
Dr Inam Ul Haq Jazbi
Number of protons = atomic number = 16
The specie must be either S (sulphur) atom or sulphur ion as it has Z = 15.
Since number of electrons given 18 which is two units
greater than the number of protons, hence the given specie must be anion with 2-
charge.
Charge on specie = Z – number of electrons = 16 – 18 = 2-
Identification of Mass number (Z+N)
Atomic mass number (A) = Z + N = 16 + 16 = 32
Result
Hence the specie is
Q3. Find the number of electrons, protons
and neutrons in fluorine atom, fluorine molecule and fluoride ion. The atomic number and mass number of
fluorine are 9 and 19 respectively.
Solution Dr Inam Ul Haq Jazbi
Q4. An isotope of atomic mass 24 had 12 neutrons in its nucleus.
What is its atomic number? Represent the isootpe in symbolic form.
Solution Dr Inam Ul Haq Jazbi
Q5. An element with three stable isotopes
has 82 protons. The separate isotopes contain 124, 125, and 126 neutrons. Identify the element
and write symbols for the isotopes.
Given:
number of protons and neutrons
Asked for:
element and atomic symbol
Strategy:
Refer to the periodic
table and use the number of protons to identify the element.
Calculate the mass
number of each isotope by adding together the numbers of protons and neutrons.
Give the symbol of each
isotope with the mass number as the superscript and the number of protons as
the subscript, both written to the left of the symbol of the element.
Solution
Part A; The element with 82
protons (atomic number of 82) is lead: Pb.
Part B;
For the first isotope, A = 82 protons + 124 neutrons = 206.
For the second isotope;
A = 82 + 125 = 207 and
For the third isotope; A = 82 + 126 = 208
The symbols for these
isotopes are
which also can also be symbolized as
Pb-206, Pb-207, and Pb-208.
Q6. What
is the mass number of a phosphorous atom with 16 neutrons?
Solution Dr
Inam Ul Haq Jazbi
Q7. How
many protons, neutrons, and electrons are in As-74 atom?
Solution Dr Inam Ul Haq Jazbi
Q8. The mass no. of
chlorine atom is 35 and the atomic no. is 17. Find the number of neutrons
present in the chlorine
atom.
Solution: Dr
Inam Ul Haq Jazbi
Mass No. (A) =
35
Atomic No (Z) =
17
Formula
Mass No (A) = Atomic No (Z) + no. of neutrons (n)
n = Mass no – Atomic no
n= 35 – 17 = 18
Therefore, the no. of neutrons present in the chlorine
atom is 18.
Q9. The number of
electrons presents in the calcium atom is 20 and the no. of neutrons present is
20. Find the atomic no.
and the mass no of the calcium atom.
Solution: Dr
Inam Ul Haq Jazbi
Given;
No. of electrons = 20,
No.
of neutrons = 20
Required;
Atomic number = ? , Mass
number = ?
Finding Atomic Number Dr
Inam Ul Haq Jazbi
Atomic number (Z) =
Number of protons = number of electrons (in neutral atom) = 20
Finding Mass Number
mass no = Atomic no (or no of protons) + No. of neutrons
= 20 + 20 =
40
Q10.The atomic nucleus of an atom has a mass number of 23 and has 12
neutrons inside its nucleus. Calculate its atomic
number. Identify the element.
Solution
Given;
Mass Number = 23,
No. of neutrons =12
Required;
Atomic number = ?
Finding Atomic Number Dr
Inam Ul Haq Jazbi
Mass no = Atomic number
(No. of protons) + No. of neutrons
Atomic number = Mass
no. – No. of neutrons = 23 – 12 = 11
Atomic No 11 is for element sodium (Na)
Q11. Two elements A and B have no. of neutrons have 20 and 14 and no.
of protons have 18 and 20. Which will have
more mass number?
Solution Dr
Inam Ul Haq Jazbi
Given
For element A; No. of protons = 18, No. of neutrons = 20
For element B; No. of protons = 20, No. of neutrons = 14
Required;
Comparing Mass numbers of A and B
= ?
Formula Dr Inam Ul Haq Jazbi
Mass number = number of
protons + number of neutrons
Finding Mass Number Dr
Inam Ul Haq Jazbi
For element A: Mass no. of element A = 18 + 20 = 38
For element B: Mass no. of element B = 20 + 14 = 34
Therefore, the Element A has more mass no. than element B.
Q12. How
many protons, neutrons, electrons and nucleons are in
Solution Dr Inam Ul Haq Jazbi
Q13. How
many protons, neutrons, electrons and nucleons are in
Solution Dr Inam Ul Haq Jazbi
Q14. How
many protons, neutrons, electrons and nucleons are in
Solution Dr Inam Ul Haq Jazbi