Important Preparation of Inorganic Compounds
Preparation of Epsom Salt/Epsomite by the action of action of dilute sulphuric acid on Mg or its compounds

Preparation of baking soda By Carbonation of Ammoniated Brine by Ammonia Solvay’s Process

Preparation of baking soda By Carbonation of Saturated Na2CO3 solution

Preparation of water glass (sodium silicate) by the Action of soda ash on Sand

Preparation white lead pigment by Chamber or Dutch Process

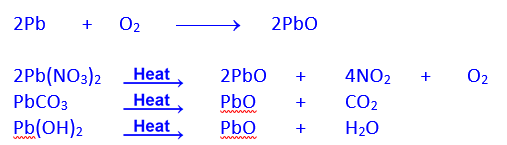
Preparation of Red lead Pigment by heating Litharge in Excess of Air

Preparation of litharge By heating Lead in Air or By Thermal Decomposition of Lead Salts
Preparation of chrome yellow pigment By action of K2CrO4 on lead acetate or lead nitrate

Preparation of chrome red pigment by the action of NaOH on Lead chromate

Preparation of Turner’s Yellow pigment by Boiling Litharge with NaCl Solution
Preparation of octahedral crystals of Alum by mixing Equi-molar solutions of sulphates of Monovalent and Trivalent Metals followed by Crystalliztion
Preparation of Bleaching Powder by Chlroiantion of Dry Slaked Lime in Hasenclever Plant
Laboratory Preparation of H2S by the Action of Dilute Acid on FeS in Kipp’s Apparatus
Industrial Preparation of H2S By Direct Union of Hydrogen gas and Sulphur at 600–650°C or By action of concentrated HCl on Stibnite (Antimony sulphide; Sb2S3)

Laboratory Preparation of H2S by the Action of Dilute Acid on FeS in Kipp’s Apparatus
Industrial Preparation of H2S By Direct Union of Hydrogen gas and Sulphur at 600–650°C or By action of concentrated HCl on Stibnite (Antimony sulphide; Sb2S3)

Preparation of (Oleum/Pyrosulphuric acid)


Preparation of Super phosphate
Preparation of Lunar caustic by dissolution of silver in dilute nitric acid

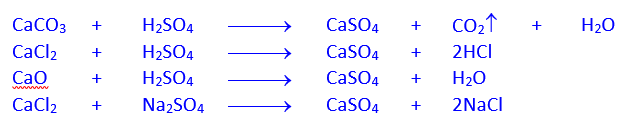
Preparation of Blue vitriol by Action of Sulphuric acid on Cu in presence of air or its compounds
Preparation of Lunar caustic by dissolution of silver in dilute nitric acid

Preparation of Blue vitriol by Action of Sulphuric acid on Cu in presence of air or its compounds
Preparation of tetraamminecopper(II) sulphate by the action of aqueous ammonia on Copper sulphate
Preparation of diamminesilver(I) nitrate by the action of aqueous ammonia on silver nitrate
Preparation of Potassium chromate by the Alkalification of potassium dichromate Using KOH or K2CO3
Preparation of K2Cr2O7 by Acidification of saturated solution of K2CrO4 with Calculated Quantity of H2SO4
Preparation of K2Cr2O7 by Addition of KCl solution into saturated solution of sodium dichromate (Na2Cr2O7)
Preparation of K2Cr2O7 by heating powdered chromite ore with excess of K2CO3 in air
Preparation of K2Cr2O7 by acidification of Potassium chromate
Preparation of Potassium Permanganate By Oxidation of potassium manganite by Chlorine or ozone

Preparation of potassium permanganate By Alkaline electrolytic Oxidation of K2MnO4

Preparation of diamminesilver(I) nitrate by the action of aqueous ammonia on silver nitrate
Preparation of Potassium chromate by the Alkalification of potassium dichromate Using KOH or K2CO3
Preparation of potassium chromate by the acidification of Potassium Chromate (K2CrO4) using Conc. H2SO4 in calculated amount
Preparation of Potassium chromate by By Alkaline Oxidation of chromium oxide by Bromine water in excess of alkali (KOH) on boiling
Preparation of K2Cr2O7 by Addition of KCl solution into saturated solution of sodium dichromate (Na2Cr2O7)
Preparation of K2Cr2O7 by heating powdered chromite ore with excess of K2CO3 in air
Preparation of K2Cr2O7 by acidification of Potassium chromate
Preparation of Potassium Permanganate from potassium manganate by fusing Mineral Pyrolusite (MnO2) with KOH in presence of air (oxidant)

Preparation of potassium permanganate By Alkaline electrolytic Oxidation of K2MnO4
















