Elimination Reactions/ β-Elimination Reactions
Definition
The reactions which involve the removal of two atoms or groups from adjacent carbon atoms of the molecule to form a multiple bond (alkene) are called elimination (E) reactions. In other words, the reaction in which β-hydrogen atom from β-carbon and an electronegative functional group (such as X- or OH-) from a-carbon are removed from organic compound yielding an unsaturated compound with a double bond between α and β-carbons is known as elimination reactions or β-elimination reactions.
e.g.
1. Dehydrohalogenation
of alkyl halides with a base
2. Acid-catalyzed dehydration of alcohols
E and SN reactions take place simultaneously
and often competition occurs.
Types of Mechanism of Elimination
Reaction (By Hughes and Ingold;
1941)
1. Bimolecular b-elimination
reaction (E2 reaction).
2. Unimolecular b-elimination reaction (E1
reaction).
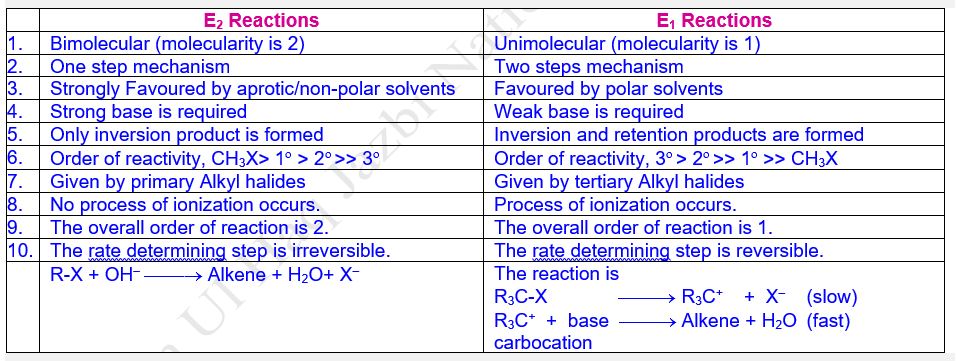
Difference between E2 and E1 Reactions
Bimolecular b-Elimination
Reaction
Definition
The single-step elimination reactions characterized
by transition state involving the removal of b-hydrogen from b-carbon
along with halide group, X‒ (F.G) group from a-carbon
of the molecule and formation of double bond between a
and b-carbons simultaneously and the reaction rate
is influenced by both the concentrations of substrate (alkyl halide) and
attacking nucleophile or base are called Bimolecular b-Elimination
Reaction; symbolized as E2 Reactions (Where E stands for elimination
and 2 stands for bimolecular).
General Representation
Example
Ethyl chloride
on heating with alcoholic potash, undergo E2 reaction, removing HX
from adjacent carbon atoms (dehydrohalogenation) forming ethene.
In E2 reactions, the removal of outgoing nucleophilic halide (X-) group and removal of b-hydrogen by the attack of strong base (e.g. OH-) take place simultaneously through transition state in which attacking nucleophile becomes partially attached to electrophilic-acidic b-hydrogen of substrate as well as the halide group (X-) is detached at the same time. This momentary unstable high-energy state is termed as transition state which readily changes to alkene by removing b-hydrogen and halide group.
As the base (B- like OH-) attacks or removes the b-hydrogen from b-carbon with simultaneous separation of halogen atom from a-carbon as halide (X-) ion and formation of double bond, take place in the single step which is also the rate-determining step in which two molecules take part, therefore, it is a bimolecular reaction. The net result is the loss of HX from alkyl halide called dehydrohalogenation which is known as b-elimination reaction. Thus one step elimination reaction involving two molecules in rate-determining step is termed as bimolecular b-elimination reaction designated as E2.
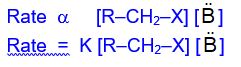
Kinetics
The rate of E2 reaction depends on the concentration of
both the alkyl halides and attacking base (B-). Thus the observed
rate for E2 reaction according to Law of Mass Action is:
Example
Primary alkyl halides
(1°) generally undergo E2 reactions.
Aprotic solvent favours E2 reaction.
Unimolecular b-Elimination (E1) Reaction
Definition
The two steps elimination reactions
characterized by slow reversible heterolytic cleavage of C – X bond of alkyl
halide into carbonium ion and halide ion followed by fast removal of a b-hydrogen
atom from b-carbon and halide group X‒ (F.G)
from a-carbon atom by the attack of base accompanied
by the formation of double bond between a and b-carbon
atoms and the reaction rate is influenced by the concentrations of only
substrate molecule (alkyl halide) are called Unimolecular b-Elimination
Reaction, symbolized as E1.
Mechanism
The
mechanism involves two steps:
Step-I (Formation of Carbonium Ion)
E1 reaction proceeds through two
steps mechanism. The first step involves the slow reversible heterolytic
fission (ionization) of C–X bond of alkyl halide into carbonium ion (R+)
and halide ion (X¯)
Step-II (Formation of Double Bond)
The second step comprises of fast removal of a
proton (H+) or b-hydrogen from the b-carbon
with the simultaneous formation of double bond between a
and b-carbon by the attack of base (OH¯).
The first step of C – X bond cleavage is slow and hence rate-determining step in which only one substrate molecule takes part, therefore, it is a unimolecular reaction. The net result is the loss of HX from alkyl halide called dehydrohalogenation which is known as b-elimination. Thus two steps elimination reaction involving only molecule in rate-determining step is termed as Unimolecular b-Elimination Reaction designated by E1.
Kinetics
The rate of
E1 reaction depends on the concentration of only Tertiary Alkyl
halide. Thus the observed rate equation for E1 reaction according to
Law of Mass Action is:
Example
Tertiary
alkyl halides (3°) generally undergo E1 reaction. Polar protic solvent favours E1 reaction.
[Secondary alkyl halides give both E2 and E1 reaction
depending upon the nature of solvent].