Allotropy and Allotropic Forms
Definition of Allotropy
Many elementary substances exist in two or
more crystalline forms differing in spatial arrangement of atoms, molecules or
ions constituting them. The occurrence of the same substance in more than one
crystalline forms is referred to as polymorphism which is exhibited by both
elements and compounds. In case of elements, polymorphism is called allotropy.
“The existence of the
same element in two or more different crystal forms in the same physical state
(i.e. without changing its state) having identical chemical properties but
distinct physical properties due to different structures or arrangement of
atoms in crystal lattice is known as allotropy (allotropia meaning variety).
The different physical forms of the same element in the same state are referred
to as allotropic modifications or allotropes.”
Reason of Allotropy
Allotropy is due to:
|
1.
|
Different crystalline structure differing
in spatial arrangement of atoms in lattice e.g. C, S, P, Sn
|
|
2.
|
Different number of atoms in the molecule
of a gas e.g. O2 and O3.
|
|
3.
|
Different molecular structure of a liquid
e.g. liquid sulphur and helium.
|
Characteristics of Allotropes
1. Allotropy is due to different arrangement of
atoms in crystal lattice.
2. Allotropic forms change into one another at a
certain temperature, transition temperature.
Transition Temperature
The allotropic forms of element have
different stabilities and unstable variety changes into the stable allotropic
form at a certain temperature called transition temperature which has fixed
value for each pair of allotropes. Thus transition temperature is the
temperature at which two crystalline forms of the same element co-exist in
equilibrium with each other.
Types
of Allotropy
Allotropy can be divided into three types:
|
1.
|
Monotropy
|
Exhibited by P (via white and red P), by C
(via graphite and diamond)
|
|
2.
|
Enantiotropy
|
Exhibited by S (via α–S and β–S)
|
|
3.
|
Dynamic allotropy
|
|
(1). Monotropy
The irreversible conversion of metastable
allotropic form of an element to its stable allotropic form at all temperatures
is called monotropy. There is no fixed transition temperature as the vapour
pressures are never equal. Monotrpy is exhibited by phosphorus via white
phosphorus and red phosphorus, by carbon via graphite (stable) and diamond
(metastable).
(2). Enantiotropy
The reversible conversion of one allotropic
form of an element into its another allotropic form at a definite temperatures
called transition temperature at which both forms coexist in dynamic
equilibrium is called enantiotropy and the allotropic forms are termed as
enantiotropes.
In some cases, one allotrope can change into
another at a definite temperature when both forms have a common vapour
pressure. This temperature is known as transition temperature. One form is stable
above this and the other form below it. When the change of one allotropic form
to the other at the transition temperature is reversible, the phenomenon is
called enantiotropy.
For example; α–sulphur on heating changes to β–sulphur at 95.5°C (transition
temperature) but on cooling below 95.5°C, β–sulphur again changes to α–sulphur. Thus α–sulphur and β–sulphur are enantiotropes.
(3). Dynamic Allotropy
The conversion of
different liquid forms of the same substance over a wide range of temperature
which can coexist in equilibrium is said to exhibit dynamic allotropy. This
form of allotropy resembles enantiotropy in that it is reversible but there is
no fixed transition temperature. The amount of each form is determined by the
temperature. The separate forms usually have different molecular formulae but
the same empirical formula.
Liquid sulphur consisting of three allotropes
Sλ, Sπ and Sμ or Sn exhibit dynamic
allotropy. These three forms of sulphur differ in molecular structure. Sλ
is S8, Sπ is S4 while formula of Sμ
is not known. The composition of equilibrium mixture at 120°C and 444.6°C (b.p.
of S) is given below:
Allotropes of Carbon
Carbon exists in
two allotropic forms:
(I). Solid Crystalline
Allotropes of Carbon
(II). Amorphous forms of
Carbon
(I). Solid
Crystalline Allotropes of Carbon
There are three solid crystalline allotropic
forms of carbon:
|
1.
|
Diamond
|
|
|
|
|
2.
|
Graphite
|
|
|
|
|
3.
|
Bucky Balls
|
(Buckminster
fullerene)
|
|
|
(II). Amorphous forms of Carbon
Amorphous forms of carbon is obtained by
heating wood, bones, sugar, starch and other organic compounds rich in carbon
in the absence of air. The amorphous forms of carbon are not considered as
allotropes of carbon because X-rays analysis revealed that they have structures
like graphite with the exception of coal (which is mined directly from natural
deposits). There are many variety amorphous carbon mainly:
Comparison of the Properties of Diamond & Graphite
(1). Diamond
Diamond is the transparent crystalline allotropic
form of carbon which is the purest, densest, hardest and highly light
reflecting form of carbon (among its various forms) having highest thermal
conductivity of any substance but showing bad electrical conductivity that
crystallizes isometricallly (cubically) consisting of carbon atoms covalently
bound by four other carbon atoms in a tetrahedral manner in three dimensional
network forming a giant macromolecule which imparts great hardness, high
stability and high melting point and permits four well-defined cleavages.
Properties
1. Pure diamond is colourless, transparent bright
crystalline solid.
2. It is the hardest natural substance known and
among various forms of carbon, diamond is the densest having a density of about
3.51 g/cm3.
3. It is a bad or non-conductor of electricity due
to lack of free electrons.
4. It has the highest thermal conductivity of any
substance.
5. It has very high melting point of about 3500°C
(3600°C or 3700°C in most books).
6. It has octahedral (cubic) crystals.
7. It has the highest refractive index (μ) of
2.45, due to which it acquires great brilliance. This property is responsible
for its value as gems. [The glitter of diamond is due to on its quality of
reflecting light. The brilliance of diamond can be increased by cutting it in
different dimensions].
8. [Pure diamond is transparent to X-rays (and
infra-red), hence X-rays is used to distinguish between imitation and pure
diamond. The
value of diamond depends upon its size and colour]. Diamonds are also blue, green,
yellow, red or black due to presence of some metal oxides as impurities,
9. The black coloured diamonds are called bort
and carbando which are of inferior qualities having great hardness and
hence are used for glass cutting, drillings and borings (grooving) of rocks and
concrete and as abrasive for polishing hard tools (surfaces).
10. The well known diamonds used as precious
stones and as jewellery are Koh-i-noor, Reagent, Victoria
11. It is quite unreactive and burns on ignition
only above 900°C to produce carbon dioxide.
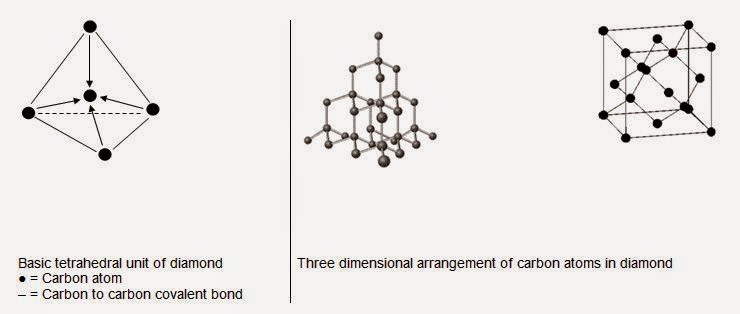
Structure and Its Properties in the Light of
Structure
Diamond is regarded
as covalent network or macromolecular solid having octahedral crystals. In
diamond, each carbon is sp3-hybridized and covalently bonded with
four other carbon atoms in tetrahedral fashion by the sp3-sp3
overlapping at an angle of 109°.5 to give basic tetrahedral units with C –C
bond length of 1.54°A and each C –C bond energy of 347 kJ/mol. These basic
tetrahedral units unite with one another indefinitely to give cubic rigid unit
cell of diamond which extends in a three-dimensional network holding thousand
of carbon atoms to form a giant three-dimensional macromolecule.
The
structure of diamond accounts for the following properties of diamond:
1. Hardness
In diamond, each carbon
atom is bonded strongly to four other carbon atoms to form basic tetrahedral
units which are united with one another in three dimensional networks to form
giant molecule or macromolecule showing cubic symmetry to give cubic unit cell
of diamond. The
C–C bond distance is 1.54°A. Thus atoms are tightly held occupying fixed positions
and it is difficult for the atoms to slide pass over the other. Due to the strength of uniformity of the
bonds, the stable rigid and closely packed tetrahedral crystal lattice, diamond
is hard.
2. High Melting Point
The bond length between carbon-carbon is
1.54°A and bond energy for each C–C bond is 347 kJ/mole. Due to strong
extensive covalent bonding extending in all directions in crystals with shorter
C – C bond length of 1.54°A and high C – C bond energy of 347 kJ/mol accounts
for its high melting point.
3. High Brilliance
The glitter of diamond
is due to its quality of reflecting light. Diamond has the highest reflecting
index of 2.45 which is a measure of brightness or brilliance of a substance.
The brilliance of diamond can be increased by cutting it in different
dimensions.
4. Bad or
Non-Conductivity of Electricity
In
diamond, all four unpaired valence electrons of each carbon atom are involved
in covalent bond formation (i.e. all the orbitals are completely filled by sharing of
four electrons) and these bonding electrons
are localized between each specific pair of carbon atoms and thus they are
unable to move freely through its crystals. The absence of free electrons or
loosely bonded electrons in diamond accounts for its bad conductivity (or
non-conductivity) of electricity.
Uses of Diamond
(1) As
gems and precious stones (for ornamental purposes) especially when they are properly
cut and polished because of their
sparkling brilliance.
(2) For cutting glasses, drilling rocks in the
form of black diamond or bort because of their great hardness.
(3) As
abrasive (in the form of its tiny fragments) for polishing hard tools.
(2). Graphite
(Plumbago or Black Lead)
Graphite
is an opaque greyish black crystalline alltropic form of carbon with metallic
sheen (lustre) and slippery or greasy nature having high electrical as well as
thermal conductivity showing greater reactivity than diamond that crystallizes
hexagonally consisting of caron atoms covalently bound by three other carbon
atoms in a trigonal manner to form basic hexagonal rings arranged in parallel
layers which are held together by weak binding forces in the form of van der
Waal’s forces forming a giant macromolecular layered lattice which accounts for
its softness and lubricating properties.
Properties
1. It is an opaque black or dark grey coloured
crystalline solid with slight or dull metallic lustre.
2. It is a very soft solid leaving black mark on
paper (because of its layered structure) and greasy to touch, (hence used as
lubricant) and is less dense than diamond having a density of 2.2 g/cm3.
3. It is good conductor of electricity (hence used
in making electrodes) due to the presence of free electrons in its crystal
lattice.
4. It has the high thermal conductivity (but less
than that of diamond).
5. It has high melting point of 3000°C (but less
than that of diamond). [In fact it sublimes at 3650°C]
6. It has hexagonal crystals.
7. It is quite stable and inert even at 2000°C and
high pressure. However, it is more reactive than diamond and burns on ignition
at 700°C to produce carbon dioxide.
Structure and Its Properties in the
Light of Structure
Graphite is regarded
as covalent network macromolecular solid having flat layered-lattice structure.
In graphite, each carbon atom is sp2 or trigonally hybridized linked
covalently to three other carbon atoms (located at the corners of an imaginary
equilateral triangles) in the same layer by sp2-sp2
overlapping making three s-bonds at an angle of
120° to give basic hexagonal rings arranged in parallel layers held together by
weak van der Waal’s forces of attraction with inter-planer distance (i.e.
distance between the two successive layers) of 3.35°A having very low
inter-layer binding energy of 3.99 kcal/mole. In hexagonal rings within a
layer, the C–C bond distance is 1.42°A (which is the bond length intermediate
to a single and a double bond with a very high C – C bond energy). The fourth valence electron of
each carbon forms the delocalized p-bond extending
uniformly over the whole layer or all carbon atoms.
The structure of graphite accounts for the
following properties of graphite:
1. Softness and low density
The loosely held flat
layered structure of graphite with weak inter-layer forces in the form of van
der Waal’s forces enabling (allowing) the layers slide over one another having
very large inter-planar distance of 3.35°A and very low inter-layer binding
energy of 3.99 kcal/mol accounts for softness, slippery texture, lubricating
property, low density and ease of cleavage. (Hence these layers can slide
easily over one another). The low density of graphite is also attributed to its
more open structure (so graphite is less dense than diamond).
2. High
Electrical Conductivity
In
parallel atomic layers of graphite, each carbon is sp2-hybridized
and has a free electron which is fully delocalized over the whole layer i.e.
spread uniformly over all carbon atoms. Due to delocalized electron graphite
conducts electricity parallel to the plane of its layers (but not perpendicular
to the layers) as this permits free movement of mobile electrons. [The
electrical conductivity of graphite is an anisotropic property; a
characteristic of crystalline solid characterized by marked variation in
intensity of certain physical properties in different directions].
3. High Melting Point
The
short bond length of 1.42°A, high bond energy of kJ/mol and strong and strong
extensive covalent bonding within the layers in graphite results in its high
melting point [but its m.p. is less than that of diamond].
4. Metallic Lustre
The surface free-floating fully delocalized
mobile valence electrons absorbing and re-radiating (re-emitting) light
accounts for its metallic luster. Due to free electrons, graphite shows metallic
lustre.
Uses of Graphite
1. Owing to high electrical conductivity, it is
used form making inert electrodes for various industrial electrolytic processes
and for dry cells.
2. Owing to
high melting points and high thermal conductivity, it is used in making
graphite lined crucibles (to withstand high temperature) which are used for
making high grade steel and other alloys.
3. Owing to soft nature, it is widely used as a
lubricant (in hot parts of the machinery where oil cannot be used), to reduce friction
in machines, bicycles chains and bearings of some motors. [Aquadag is a
colloidal solution of graphite in water with little tannic acid, C76H52O46
and much used as lubricant].
4. It is used in the manufacture of lead
pencils when mixed with clay. [For this purpose, a variable composition of
graphite and fine clay is used. Lead pencils are made by mixing graphite with
20-60% clay. The proportion of clay to graphite in a pencil determines the
hardness of a pencil. Pencil becomes hard by increasing the amount of clay.
Different grades of pencils like H, 2H, HB, 2B containing different amount of
clay have been used].
5. It is used a neutron moderator in nuclear
reactions.
6. It is used a black pigment in paints.
(3). Bucky
Balls
In 1985, a new type of allotropic forms of
carbon was discovered by the vapourized graphite by two English researchers who
named it bucky balls or Buckminster fullerene [after an architect Buckminster,
who designed a bucky ball shaped building in Montreal , Canada
It has been found that in bucky balls, carbon
atoms is about 60 forming C60 molecules because the mass spectrum
peaks correspond to cluster of carbon atoms as molecules of 60 carbon atoms (C60)
which fold around or arranged in a hollow cage like spherical structure forming
a ball like a football or soccer ball with highly symmetrical structure. The
carbon atoms join together to form pentagon and hexagon structures.
Unlike diamond and graphite, the new
molecular form of carbon; bucky balls can be dissolved in organic solvents.
Bucky balls act as a semi-conductors and lubricants.