Proteins (its definition,
importance and types)
Definition of Proteins
All proteins yield amino acids which
are generally a-amino
carboxylic acids of different molecular sizes, upon complete hydrolysis. Thus,
amino acids are the basic constructing units of proteins. The human body
probably contains at least 10,000 different kinds of proteins. The
proteins constitute 50% of the dry weight of the living cells (that is
why they have given a name of proteins derived from the Greek work proteios
meaning of prime importance). Proteins are present in all living organisms and
are found in muscles, skin, hair, nails and other tissues that make up the bulk
of the body’s non-bony structure.
Proteins (derived from Proteios meaning first) are the complex nitrogenous long chains polymeric organic
macromolecules of very high molecular weight (34000- 5000000 dalton
or 17500 -6,000,000 Da) which are giant
linear condensation biopolymers of a-amino acid monomers (ranging from 200-6,0000)
interlocked or linked together through peptide linkages (acid-amide bonds) through
condensation polymerization with the elimination of water molecules. In other
words, Proteins are high molecular weight organic polypeptides which yield a- amino acids upon complete hydrolysis. [The aggregation of two or more amino acids linked
together through peptide bond is called Peptide. A dipeptide is a condensation
product of two amino acids containing a single peptide bond while a tripeptide
is a condensation product of three amino acids containing two peptide bonds. A
condensation product of 10 or more amino acids is called Polypeptide]. A
polypeptide of high molecular weight more than 10,000 da containing more than
200 amino acids is called protein.
All proteins contain the elements C,
H, O, N and S. Other elements like P and traces of other elements such as
Ca, Fe, Mn, Cu, Zn and I may be the essential constituents of some specialized proteins.
e.g. Casein (milk protein) contains Ca and P, haemoglobin (iron containing
protein).
Bio-medical Importance of Proteins
1.
|
Building and maintenance of body tissues
|
(Structural
proteins)
|
2.
|
Catalytic Function/ Biochemical Catalysis
|
(Enzymatic proteins or Enzymes)
|
3.
|
Regulation
of metabolism
|
(Hormonal proteins like insulin)
|
4.
|
Contraction
of muscles
|
(Contractile
proteins like Actin and myosin filaments)
|
5.
|
Storage
Function
|
(Storage
proteins like Lipoproteins, ferritin)
|
6.
|
Protective
Function
|
(Immunal proteins like Antibodies like
globulins and γ-globulins)
|
7.
|
Respiratory
Function/ Oxygen carrier
|
(Haemoglobin, cytochrome, haemocyanin, myoglobin)
|
8.
|
Genetic
Regulation
|
(Genetic
proteins or nucleoproteins like DNA and RNA)
|
Classification of Proteins Based on Physio-Chemical Properties
Proteins are of three types:
1.
|
Simple proteins
|
;
|
Consist
of only amino acids or their derivatives e.g. albumin, globulin
|
(a) Albumins
|
(e.g. egg
albumin, serum albumin, milk albumin/lactalbumin, Wheat albumin, leucosin
|
||
(b) Globulins
|
(e.g. egg globulin,
serum globulin, myosin/muscle globulin, amandin in almonds
|
||
(c) Glutelins
|
(e.g.
|
||
(d) Gliadin/Prolamine
|
(e.g. hordein
in barley, zein in maze corn, gliadin in wheat)
|
||
(e) Albuminoids
|
(e.g. casein
& collagen in tendons, keratin in hair, horn, nails)
|
||
2.
|
Conjugated proteins
|
;
|
Consist of simple proteins with prosthetic group
e.g. Haemoglobin,
|
(a) Glyco-proteins
|
|||
(b) Lipo-proteins
|
|||
(c) Nucleo-proteins
|
|||
(d) Phospho-proteins
|
|||
(e) Chromo-proteins
|
|||
3.
|
Derived Proteins
|
;
|
Degraded
proteins e.g. proteoses,
polypeptides, peptides, peptones.
|
1).Simple Proteins
These are the simplest proteins
which upon hydrolysis yield only amino acids and their derivatives. e.g. egg
albumin, serum albumin of blood, and milk albumin (lactalbumin) etc.
2). Conjugated Proteins
These are the proteins which consist
of simple proteins with non-proteinous substance called prosthetic group. e.g.
Haemoglobin, Chlorophyll.
3). Derived Proteins
These are not naturally occurring proteins and are obtained by
degradation of proteins by acids, alkalis, heat, enzymes or biochemical action.
e.g. peptones, proteoses, polypeptides, peptides.
Definition and General Formula
Amino acids are the basic building blocks of proteins. Amino acids are bifunctional (polyfunctional) organic acids having both an acidic carboxyl group (-COOH) and a basic amino group (-NH2 or >NH) along with distinct side chain R (which is different for different amino acids). Almost all the naturally occurring amino acids are a-amino acids in which amino group is attached to a-carbon atom (relative to the carboxyl group). They have following general formula:
Where R may be hydrogen, a straight or branched chain alkyl group or an aryl group.
The amino group in amino acids may be present at any carbon atom other than that of carboxyl group containing carbon (i.e. a– carbon). Based on whether the amino group is present on the a, β or γ - carbon atom relative to the carboxyl group, amino acids are referred to as a, β and γ–amino acids respectively.
Bio-medical Importance of Amino Acids
1.
|
Protein synthesis
| |
2.
|
Hormones synthesis
| |
3.
|
Energy Source
| |
4.
|
Regulation of metabolism
|
Types of Amino Acids Based on their Acidity or Alkanity
1.
|
Neutral amino acids
|
;
|
contain one –NH2 & one –COOH group.
|
e.g.
|
Glycine, alanine, Valine, Leucine
|
2.
|
Acidic amino acids
|
;
|
contain more than one –COOH groups.
|
e.g.
|
Aspargine, Glutamine
|
3.
|
Basic amino acids
|
;
|
contain more than one –NH2 groups
|
e.g.
|
Lysine, Arginine.
|
Essential Amino Acids and Non-essential Amino Acids
About 20 amino acids have been identified as the constituents of most of the animal and plant proteins. Out of 20 amino acids which are required for protein synthesis, the human body can synthesize only 10 and such amino acids are called Non-essential amino acids. e.g. Glycine, alinine, proline, aspartic acid, glutamic acid, tyrosine, serine, cysteine, aspargine.
The ten amino acids that are not synthesized by human body and hence are needed to be provided in the diet for proper health and growth are called Essential Amino Acids. [They are 10 for infants and 8 for adult human being]. They include Valine, Leucine, Isoleucine, Lysine, Methionine, Threonine, Arginine, Tryptophan, Phenylalanine, and Histidine (last two are needed for infants).
Zwitterion
The dipolar but overall electrically neutral charged ion with positive as well as negative ends within the same molecule is termed as Zwitterion (German; two ions). The dipolar ionic structure is also called internal salt. All a–amino acids exist largely as dipolar ionic forms or Zwitterions in solution that is formed when the proton goes from the acidic carboxylic group (on ionization) to basic amino group (Lewis base).
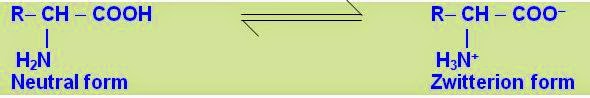 | |||||
Due to the Zwitter ions form, amino acids are soluble in water but insoluble in organic solvents. The Zwitterion formation makes amino acids amphoteric and allows them to donate or accept proton from the medium or solvent in which they are dissolved.
Peptide Linkage
The acid-amide (-CO -NH -) bond through which amino acids are linked in proteins by eliminating a water molecule is called Peptide Linkage. This linkage is formed by the removal of a water molecule b/w an -NH2 group of an amino acid and -COOH group of another. The product formed from condensation of two amino acids containing a single peptide linkage is called Dipeptide. The product formed from condensation of three amino acids containing two peptide bonds is called Tripeptide.



