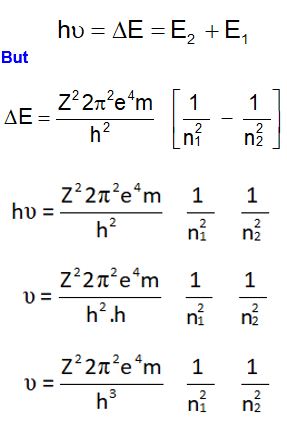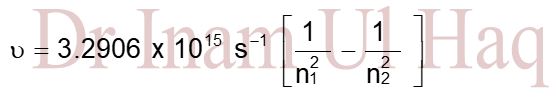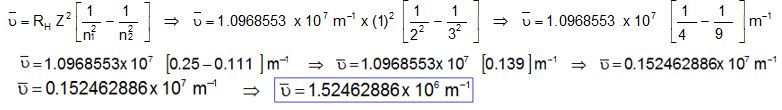Derivation
of Radius of the nth Bohr’s Orbit for Hydrogen-Like Atoms (Single
electron system)
Assumptions for Simple Atom
To derive an expression for radius, consider a hydrogen atom with atomic number equal to Z consisting of a single electron with charge –e and mass m revolving around the nucleus of charge +Ze (+e is charge of proton) with a tangential velocity v in an stationary orbit of radius r.
Electrostatic force of
Attraction/Centripetal Force and Centrifugal force
At uniform circular equilibrium motion, these two opposite forces must be
equal to each other i.e.
Determination
of v2 of electron by using Bohr’s Postulate
According to Bohr’s postulate, angular momentum of electron revolving
around the nucleus is an integral multiple of h/2p.
Calculation
of Radius (r)
Substituting the value v2 from equation (ii) in
equation (i)
Calculation of nth Bohr’s orbit (rn)
The above equation shows that radius of orbit is directly proportional to
the square of the principal quantum numbers (r α n2 i.e. 1, 2, 3, ……..)
and inversely proportional to atomic number. As the value of n increases, the radius
of the orbit will increase.
Derivation of Energy of the nth Bohr’s Orbit
Basic of Derivation
The total energy of an electron revolving in any orbit around the nucleus is given by,
Calculation of K.E
The K.E. of electron with mass m revolving around the nucleus with velocity v is given by the following expression;
Now the centrifugal and centripetal forces upon the revolving electron are given as:
Now the centrifugal and centripetal forces upon the revolving electron are given as:
At uniform circular equilibrium motion, these two opposite forces must be equal to each other i.e.
Calculation of P.E Using Definition of Work

Calculation of Total Energy


Put the value of “r” from equation (iii) to equation (ii)

Conclusion
If total energy = - x
Then
KE = + x
PE = - 2x
Expression for ∆E of Electronic Transition between Orbits
Calculation of ∆E
To calculate the energy emitted by atom in the form of radiation
when an electron jumps from a higher energy state n1; let us make
use of the postulate of Bohr’s model; according to which:
Expression for Frequency of Electronic Transition between Orbits
To calculate the frequency of emitted radiations (or photons there in); let us make use of the Bohr’s postulate; according to which:
Expression for Wave Number and Wave Length of Radiation
Q1. Calculate the wave number and wavelength of Balmer series of hydrogen spectrum in which electron jumps from orbit 3 to orbit 2.
Solution
Calculation of Wave Number
Here;
RH = 1.0968553 x 107 m-1
n1 = 2
n2 = 3
Z = 1
Calculation of Wave Length
Q2. What is the wavelength and wave number of radiation that is emitted when a hydrogen atom undergoes a transition from orbit 3 to orbit 1.
Solution
Calculation of Wave Number
RH = 1.0968553 x 107 m-1
n1 = 1
n2 = 3
 |
Q3.Calculate the ratio of radius of second and third orbit of hydrogen atom. (Bohr’s radius = 0.529ºA)
Solution
Solution
Q1.Calculate the energy of the electron in theground state and first excited state of the hydrogen atom
Solution
Part (A)
Z for H = 1
n for ground state = 1
|
|
| ||
|
|
|
|
|
|
|
|
|